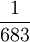SI base units
edit
| Name
| Symbol
| Measure
| Definition
| Historical Origin/Justification
|
| metre or meter
| m
| length
| The unit of length is equal to the length of the path traveled by light in a vacuum during the time interval of 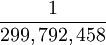 of a second. Equal to 100 cm (39.37 inches) Defined by: 17th CGPM (1983) Resolution 1, CR 97 of a second. Equal to 100 cm (39.37 inches) Defined by: 17th CGPM (1983) Resolution 1, CR 97
| 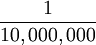 of the distance from the Earth's equator to the North Pole measured through Paris. of the distance from the Earth's equator to the North Pole measured through Paris.
|
| kilogram
| kg
| mass
| The unit of mass is equal to the mass of the international prototype kilogram (a platinum-iridium cylinder) kept at the Bureau International des Poids et Mesures (BIPM), Sèvres, Paris (1st CGPM (1889), CR 34-38). Note that the kilogram is the only base unit with a prefix. See the kilogram article for an alternative definition.
| The mass of one litre of water. Kilogram was originally named "grave" and symbolized g. The gram is defined as a derived unit, equal to 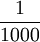 of a kilogram; prefixes such as mega are applied to the gram, not the kg; e.g. Gg, not Mkg. It is also the only unit still defined by a physical prototype instead of a measurable natural phenomenon. of a kilogram; prefixes such as mega are applied to the gram, not the kg; e.g. Gg, not Mkg. It is also the only unit still defined by a physical prototype instead of a measurable natural phenomenon.
|
| seconds, minutes, hours
| s, min, hrs
| time
| The unit of time is the duration of exactly 9,192,631,770 periods of the radiation corresponding to the transition between two hyperfine levels of the ground state of the caesium-133 atom at a temperature of 0 K. Defined by: 13th CGPM (1967-1968) Resolution 1, CR 103
| The day is divided in 24 hours, each hour divided in 60 minutes, each minute divided in 60 seconds.
A second is 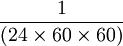 of the day of the day
|
| ampere
| A
| electric current
| The ampere is the unit of electric current. The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 metre apart in vacuum, would produce between these conductors a force equal to 2·10–7 newton per metre of length.
|
|
| kelvin
| K
| thermodynamic temperature
| The unit of thermodynamic temperature (or absolute temperature) is the fraction 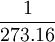 (exactly) of the thermodynamic temperature at the triple point of water. Defined by: 13th CGPM (1967) Resolution 4, CR 104 (exactly) of the thermodynamic temperature at the triple point of water. Defined by: 13th CGPM (1967) Resolution 4, CR 104
| Historically, the Celsius scale was used before the kelvin. 1 degree Celsius (or degree centigrade) = 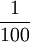 of the difference between the boiling and freezing points of water. The kelvin scale uses the degree Celsius for its unit increment. of the difference between the boiling and freezing points of water. The kelvin scale uses the degree Celsius for its unit increment.
|
| mole
| mol
| quantity of matter (mass/mass)
| A mole is the quantity of substance that contains the same number of elementary entities (atoms, molecules, ions, electrons or particles, depending on the substance) as there are atoms in 0.012 kilograms of pure carbon-12; this number (NA) is approximately equal to 6.0221415(10) * 1023 mol-1 (2002 CODATA).
| one gram per atomic mass unit
|
| candela
| cd
| luminous intensity
| The unit of luminous intensity is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 * 1012 hertz and that has a radiant intensity in that direction of 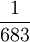 watt per steradian. Defined by: 16th CGPM (1979) Resolution 3, CR 100 watt per steradian. Defined by: 16th CGPM (1979) Resolution 3, CR 100
| the candlepower
|
Pages in category "SI base units"
There are 9 pages in this section of this category.


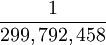 of a second. Equal to 100 cm (39.37 inches) Defined by: 17th CGPM (1983) Resolution 1, CR 97
of a second. Equal to 100 cm (39.37 inches) Defined by: 17th CGPM (1983) Resolution 1, CR 97
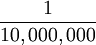 of the distance from the Earth's equator to the North Pole measured through Paris.
of the distance from the Earth's equator to the North Pole measured through Paris.
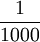 of a kilogram; prefixes such as mega are applied to the gram, not the kg; e.g. Gg, not Mkg. It is also the only unit still defined by a physical prototype instead of a measurable natural phenomenon.
of a kilogram; prefixes such as mega are applied to the gram, not the kg; e.g. Gg, not Mkg. It is also the only unit still defined by a physical prototype instead of a measurable natural phenomenon.
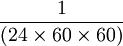 of the day
of the day
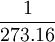 (exactly) of the thermodynamic temperature at the
(exactly) of the thermodynamic temperature at the 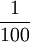 of the difference between the boiling and freezing points of water. The kelvin scale uses the degree Celsius for its unit increment.
of the difference between the boiling and freezing points of water. The kelvin scale uses the degree Celsius for its unit increment.