To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Dinitrogen trioxide
Dinitrogen trioxide is the chemical compound with the formula N2O3. This pale blue liquid is one of binary nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21°C (−6°F):
Product highlightDinitrogen trioxide is only isolable at low temperatures, i.e. in the liquid and solid phases. At higher temperatures the equilibrium favors the constituent gases. Kdiss = 193 kPa (25 °C).[1] Structure and bondingDinitrogen trioxide has an unusually long N−N bond at 186 pm. Whereas N−N bonds are more often similar to that in hydrazine (145 pm), some other oxides of nitrogen do possess long N−N bonds, including dinitrogen tetroxide (175 pm). The N2O3 molecule is planar and exhibits Cs symmetry. The dimensions displayed below come from microwave spectroscopy of low-temperature, gaseous N2O3: It is the anhydride of the unstable nitrous acid (HNO2), and produces it when mixed into water. An alternative structure might be anticipated for the true anhydride, i.e. O=N-O-N=O, but this isomer is not observed. If the nitrous acid is not then used up quickly, it decomposes into nitric oxide and nitric acid. Nitrite salts are sometimes produced by adding N2O3 to solutions of bases:
Nitrogen Trioxide = NO3 References
Categories: Oxides | Nitrogen compounds | Acidic oxides |
||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Dinitrogen_trioxide". A list of authors is available in Wikipedia. | ||||||||||||||||||||||||||||||||||


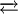 N2O3
N2O3





