To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Standard electrode potentialIn electrochemistry, the standard electrode potential, abbreviated Eo, is the measure of individual potential of a reversible electrode (at equilibrium) at standard state, which is with solutes at an effective concentration of 1 mol/kg, and gases at a pressure of 1 bar. The values are most often tabularized at 25 °C. The basis for an electrochemical cell such as the galvanic cell is always a redox reaction which can be broken down into two half-reactions: oxidation at anode (loss of electron) and reduction at cathode (gain of electron). Electricity is generated due to electric potential difference between two electrodes. This potential difference is created as a result of the difference between individual potentials of the two metal electrodes with respect to the electrolyte. Although the overall potential of a cell can be measured, there is no simple way to accurately measure the individual electrode/electrolyte potentials in isolation. The electric potential also varies with temperature, concentration and pressure. Since the oxidation potential of a half-reaction is the negative of the reduction potential in a redox reaction, it is sufficient to calculate either one of the potentials. Therefore, standard electrode potential is commonly written as standard reduction potential. Product highlight
Calculation of standard electrode potentialsTo overcome the difficulty of measuring individual potential of an electrode, an electrode of unknown reduction potential can be paired with a reference electrode of a known potential. The ultimate reference is the standard hydrogen electrode (SHE) whose potential is defined to be exactly zero volts at all temperatures. For example, to measure the standard reduction potential of zinc metal electrode, an electrochemical cell can be built with a zinc metal electrode (e.g. a zinc electrode immersed in 1 M ZnSO4 solution) as the anode. The anode half-reaction is then:
The SHE is used as the cathode and overall cell can be written in shorthand form:
Since the reduction half-reaction has a potential of zero, the EMF of the cell, Eocell, corresponds to the potential of the zinc metal electrode because:
Since the electrode potentials are conventionally defined as reduction potentials, the sign of the potential for the metal electrode being oxidized must be reversed when calculating the overall cell potential. Note that the electrode potentials are independent of the number of electrons transferred and so the two electrode potentials can be simply combined to give the overall cell potential even if different numbers of electrons are involved in the two electrode reactions (more care is required if combining electrode potentials to derive a third electrode potential). Standard reduction potential tableSince the values are given in their ability to be reduced, the bigger the standard reduction potentials, the easier they are to be reduced, in other words, they are simply better oxidizing agents. For example, F2 has 2.87 V and Li+ has -3.05 V. F2 reduces easily and is therefore a good oxidizing agent. In contrast, Li+ would rather undergo oxidation (hence a good reducing agent). Thus Zn2+ whose standard reduction potential is -0.76 V can be oxidized by any other electrode whose standard reduction potential is greater than -0.76 V (eg. H+(0 V), Cu2+(0.16 V), F2(2.87 V)) and can be reduced by any electrode with standard reduction potential less than -0.76 V (eg. H2(-2.23 V), Na+(-2.71 V), Li+(-3.05 V)). In a galvanic cell, where a spontaneous redox reaction drives the cell to produce an electric potential, Gibbs free energy ΔGo must be negative, in accordance with the following equation:
where n is number of moles of electrons per mole of products and F is the Faraday constant, ~96485 C/mol. As such, the following rules apply:
Thus in order to have a spontaneous reaction (-ΔGo), Eocell must be positive, where:
where Eoanode is the standard potential at the anode (reverse the sign of the standard reduction potential value for the electrode) and Eocathode is the standard potential at the cathode as given in the table of standard electrode potential. Non-standard conditionThe standard electrode potentials are given at standard conditions. Yet in most cases, real cells may operate under non-standard conditions. Given the standard potential of the half-cell, its potential at non-standard effective concentrations can be calculated using the Nernst equation:
The values of E0 depend on temperature (except for SHE, for which the potential has been, arbitrarily, declared 0 at all temperatures) and are normally referenced to the SHE at the same temperature. For condensed phases, they are also expected to depend somewhat on pressure (see the article on equilibrium constant). For example, the standard electrode potential for Ni/NiO redox couple has been well studied because such a solid has applications in high-temperature pseudo-reference electrodes (when enclosed inside an yttrium-stabilized zirconia ceramic membrane). The standard potential of Ni/NiO has been correlated for temperatures between 0 and 400 °C to be approximately[1]:
where E0 is in volts, and T is in degrees Celsius. In biochemistry, potentials are usually defined for pH 7, with the standard potential under these conditions being Eo' - also referred to as the mid-point potential or Em,7 because it is the potential at which the concentrations of the oxidised and reduced forms of the redox pair are equal. The actual redox potential for a pair at a given pH of x (Eh, pH = x) is related to the midpoint potential by:
See alsoFurther reading
References
|
|||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Standard_electrode_potential". A list of authors is available in Wikipedia. |





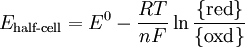
![E_{h,pH=x} = E_{m,pH=x} - \frac{2.3RT}{nF}\log_{10}\frac{[\text{red}]}{[\text{oxd}]}](images/math/7/b/7/7b78c498c4c583437710a1c010b4aa49.png)


