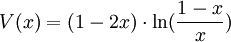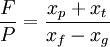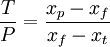To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Enriched uranium
Enriched uranium is a kind of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Natural uranium is 99.284% 238U isotope, with 235U only constituting about 0.711 % of its weight. However, 235U is the only isotope existing in nature (in any appreciable amount) that is fissionable by thermal neutrons. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. The International Atomic Energy Agency attempts to monitor and control enriched uranium supplies and processes in its efforts to ensure nuclear power generation safety and curb nuclear weapons proliferation. During the Manhattan Project enriched uranium was given the codename oralloy, a shortened version of Oak Ridge alloy, after the location of the plants where the uranium was enriched. The term oralloy is still occasionally used to refer to enriched uranium. There are about 2,000 metric tons of highly enriched uranium in the world,[1], produced mostly for nuclear weapons, naval propulsion, and smaller quantities for research reactors. The 238U remaining after enrichment is known as depleted uranium (DU), and is considerably less radioactive than even natural uranium, though still extremely dense. It is useful for armour, penetrating weapons, and other applications requiring very dense metals, though at the present time, only 5% of it is put to any use; the rest remains in storage at the enrichment facilities. Product highlight
GradesSlightly enriched uranium (SEU)
Slightly enriched uranium (SEU) has a 235U concentration of 0.9% to 2%. This new grade is being used to replace natural uranium (NU) fuel in some heavy water reactors like the CANDU. Costs are lowered because less uranium and fewer bundles are needed to fuel the reactor. This in turn reduces the quantity of used fuel and its subsequent waste management costs. Recovered uranium (RU) is a variation of SEU. It is based on a fuel cycle involving used fuel recovered from light water reactors (LWR). The spent fuel from a LWR typically contains more U-235 than natural uranium, and therefore could be used to fuel reactors that customarily use natural uranium as fuel. Low-enriched uranium (LEU)Low-enriched uranium (LEU) has a lower than 20% concentration of 235U. For use in commercial light water reactors (LWR), the most prevalent power reactors in the world, uranium is enriched to 3 to 5 % 235U. Fresh LEU used in research reactors is usually enriched 12% to 19.75% U-235, the latter concentration being used to replace HEU fuels when converting to LEU. Highly enriched uranium (HEU)Highly enriched uranium (HEU) has a greater than 20% concentration of 235U or 233U. The fissile uranium in nuclear weapons usually contains 85% or more of 235U known as weapon(s)-grade, though for a crude, inefficient weapon 20% is sufficient (called weapon(s)-usable); some argue that even less is sufficient, but then the critical mass required rapidly increases. However, judicious use of implosion and neutron reflectors can enable construction of a weapon from a quantity of uranium below the usual critical mass for its level of enrichment, though this would likely only be possible in a country which already had extensive experience in developing nuclear weapons. The presence of too much of the 238U isotope inhibits the runaway nuclear chain reaction that is responsible for the weapon's power. The critical mass for 85% of highly enriched uranium is about 50 kilograms, which at normal density would be a sphere less than 7 inches in diameter. HEU is also used in fast neutron reactors as well as in naval reactors, where it contains at least 50% 235U, but typically does not exceed 90%. The Fermi-1 commercial fast reactor prototype used HEU with 26.5% 235U. For criticality experiments, enrichment of uranium to over 97% has been accomplished.[2] MethodsIsotope separation is a difficult and energy intensive activity. Enriching uranium is difficult because the two isotopes have identical chemical properties, and are very similar in weight: 235U is only 1.26% lighter than 238U. Several production techniques applied to enrichment have been used, and several are under investigation. In general these methods exploit the slight differences in atomic weights of the various isotopes. Some work is being done that would use nuclear resonance; however it is not certain if any of these processes have been scaled up to production.[citation needed] A feature common to all large-scale enrichment schemes is that they employ a number of identical stages which produce successively higher concentrations of 235U. Each stage concentrates the product of the previous step further before being sent to the next stage. Similarly, the tailings from each stage are returned to the previous stage for further processing. This sequential enriching system is called a cascade. Thermal diffusionThermal diffusion utilizes the transfer of heat across a thin liquid or gas to accomplish isotope separation. The process exploits the fact that the lighter 235U gas molecules will diffuse toward a hot surface, and the heavier 238U gas molecules will diffuse toward a cold surface. The S-50 plant at Oak Ridge, Tennessee was used during World War II to prepare feed material for the EMIS process. It was abandoned in favor of gaseous diffusion. Gaseous diffusionGaseous diffusion is a technology used to produce enriched uranium by forcing gaseous uranium hexafluoride (Hex) through semi-permeable membranes. This produces a slight separation between the molecules containing 235U and 238U. Throughout the Cold War, gaseous diffusion played a major role as a uranium enrichment technique, though it has now been almost completely replaced by newer methods. Gas centrifugeThe gas centrifuge process uses a large number of rotating cylinders in series and parallel formations. This rotation creates a strong centrifugal force so that the heavier gas molecules containing 238U move toward the outside of the cylinder and the lighter gas molecules rich in 235U collect closer to the center. It requires far less energy to achieve the same separation than the older gaseous diffusion process, which it has largely replaced. Zippe centrifugeThe Zippe centrifuge is an improvement on the standard gas centrifuge, the primary difference being the use of heat. The bottom of the rotating cylinders is heated, producing convection currents that move the 235U up the cylinder, where it can be collected by scoops. This improved centrifuge design is used commercially by Urenco to produce nuclear fuel and was used by Pakistan in their nuclear weapons program. The Zippe-type technology was transferred by Pakistani scientist Abdul Qadeer Khan to North Korea, Libya and Iran, allowing them to develop their nuclear industries and to potentially develop nuclear weapons. (In October, 2006, North Korea announced a successful test of a nuclear weapon, although it is clearly known that the fuel for this weapon was not produced from gas centrifuge technology, and the U.N. confirmed it. Iran denies having a nuclear weapons program, however several nations claim that Iran intends to use its civilian enrichment program to make actual weapons.) Aerodynamic processesAerodynamic enrichment processes include the Becker Jet Nozzle Techniques developed by EW Becker and associates and the vortex tube separation process. These aerodynamic separation processes depend upon diffusion driven by pressure gradients, as does the gas centrifuge. In effect, aerodynamic processes can be considered as non-rotating centrifuges. Enhancement of the centrifugal forces is achieved by dilution of UF6 with hydrogen or helium as a carrier gas achieving a much higher flow velocity for the gas than could be obtained using pure uranium hexafluoride. The Uranium Enrichment Corporation of South Africa (UCOR) developed and deployed the Helikon vortex separation process based on the vortex tube and a demonstration plant was built in Brazil by NUCLEI, a consortium led by Industrias Nucleares do Brasil that used the separation nozzle process. However both methods have high energy consumption and substantial requirements for removal of waste heat; neither is currently in use. Electromagnetic isotope separation
In the electromagnetic isotope separation process (EMIS), metallic uranium is first vaporized, and then ionized to positively charged ions. The cations are then accelerated and subsequently deflected by magnetic fields onto their respective collection targets. A production-scale mass spectrometer named the Calutron was developed during World War II that provided some of the 235U used for the Little Boy nuclear bomb, which was dropped over Hiroshima in 1945. Properly the term 'Calutron' applies to a multistage device arranged in a large oval around a powerful electromagnet. Electromagnetic isotope separation has been largely abandoned in favour of more effective methods. Laser processesLaser processes are a possible third-generation technology promising lower energy inputs, lower capital costs and lower tails assays, hence significant economic advantages. Atomic Vapor Laser Isotope Separation (AVLIS) is a method by which specially tuned lasers are used to separate isotopes of uranium using selective ionization of hyperfine transitions. The technique uses lasers which are tuned to frequencies that ionize a 235U atom and no others. The positively-charged 235U ions are then attracted to a negatively-charged plate and collected. A second method of laser separation is known as molecular laser isotope separation (MLIS). In this method, an infrared laser is directed at uranium hexafluoride gas, exciting molecules that contain a 235U atom. A second laser frees a fluorine atom, leaving uranium pentafluoride which then precipitates out of the gas. An Australian development which is molecular and utilises UF6 called SILEX (Separation of Isotopes by Laser EXcitation) apparently is “fundamentally completely different from what has been tried elsewhere" according to Silex Systems Ltd.[1], the developer. Details of the process are currently not available. After a protracted development process involving U.S. enrichment company USEC acquiring and then relinquishing commercialization rights to the technology, General Electric has signed a commercialization agreement with Silex Systems in 2006 (see here). None of these processes is yet ready for commercial use, though SILEX is well advanced. Chemical methodsOne chemical process has been demonstrated to pilot plant stage but not used. The French CHEMEX process exploited a very slight difference in the two isotopes' propensity to change valency in oxidation/reduction, utilising immiscible aqueous and organic phases. An ion-exchange process was developed by the Asahi Chemical Company in Japan which applies similar chemistry but effects separation on a proprietary resin ion-exchange column. Plasma separationPlasma separation process (PSP) describes a technique potentially more efficient at uranium-enrichment that makes use of superconducting magnets and plasma physics. In this process, the principle of ion cyclotron resonance is used to selectively energize the 235U isotope in a plasma containing a mix of ions. The French developed their own version of PSP, which they called RCI. Funding for RCI was drastically reduced in 1986, and the program was suspended around 1990, although RCI is still used for stable isotope separation. Separative work unitThe Separative Work Unit (SWU) is a function of the amount of uranium processed, the composition of the starting material, and the degree to which it is enriched; it is proportional to the total machine operation time required to achieve this. Separative work is expressed in SWUs, kg SW, or kg UTA (from the German Urantrennarbeit )
The unit is strictly: Kilogram Separative Work Unit, and is indicative of the energy used in enrichment, when feed, tails and product quantities are expressed in kilograms. The work WSWU necessary to separate a mass F of feed of assay xf into a mass P of product assay xp, and tails of mass T and assay xt is expressed in terms of the number of separative work units needed, given by the expression where The feed to product ratio is given by the expression whereas the tails to product ratio is given by the expression If, for example, you begin with 100 kilograms (220 pounds) of NU, it takes about 61 SWU to produce 10 kilograms (22 pounds) of LEU in 235U content to 4.5%, at a tails assay of 0.3%. The number of Separative Work Units provided by an enrichment facility is directly related to the amount of energy that the facility consumes. Modern gaseous diffusion plants typically require 2,400 to 2,500 kilowatt-hours (8,600 to 9,000 megajoules or 9 gigajoules) of electricity per SWU while gas centrifuge plants require just 50 to 60 kilowatt-hours (180 to 220 MJ) of electricity per SWU. Example: A large nuclear power station with a net electrical capacity of 1300 MW requires about 25,000 kg of LEU annually with a 235U concentration of 3.75%. This quantity is produced from about 210,000 kg of NU using about 120,000 SWU. An enrichment plant with a capacity of 1000 kSWU/yr is, therefore, able to enrich the uranium needed to fuel about eight large nuclear power stations. Cost Issues In addition to the Separative Work Units provided by an enrichment facility, the other important parameter that must be considered is the mass of NU that is needed in to order to yield a desired mass of enriched uranium. As with the number of SWUs, the amount of feed material required will also depend on the level of enrichment desired and upon the amount of 235U that ends up in the depleted uranium. However, unlike the number of SWUs required during enrichment which increases with decreasing levels of 235U in the depleted stream, the amount of NU needed will decrease with decreasing levels of 235U that end up in the DU. For example, in the enrichment of LEU for use in a light water reactor it is typical for the enriched stream to contain 3.6% 235U (as compared to 0.7% in NU) while the depleted stream contains 0.2% to 0.3% 235U. In order to produce one kilogram of this LEU it would require approximately 8 kilograms of NU and 4.5 SWU if the DU stream was allowed to have 0.3% 235U. On the other hand, if the depleted stream had only 0.2% 235U, then it would require just 6.7 kilograms of NU, but nearly 5.7 SWU of enrichment. Because the amount of NU required and the number of SWUs required during enrichment change in opposite directions, if NU is cheap and enrichment services are relatively more expensive, then the operators will typically choose to allow more 235U to be left in the DU stream whereas if NU is relatively more expensive and enrichment is less so, then they would choose the opposite....
DownblendingThe opposite of enriching is downblending; Surplus HEU can be downblended to LEU to make it suitable for use in commercial nuclear fuel. The HEU feedstock can contain unwanted uranium isotopes: 234U is a minor isotope contained in natural uranium; during the enrichment process, its concentration increases but remains well below 1%. High concentrations of 236U is a byproduct from irradiation in a reactor and may be contained in the HEU, depending on its manufacturing history. HEU reprocessed from nuclear weapons material production reactors (with an 235U assay of approx. 50%) may contain 236U concentrations as high as 25%, resulting in concentrations of approximately 1.5% in the blended LEU product. 236U is a neutron poison; therefore the actual 235U concentration in the LEU product must be raised accordingly to compensate for the presence of 236U. The blendstock can be NU, or DU, however depending on feedstock quality, SEU at typically 1.5 wt% 235U may used as a blendstock to dilute the unwanted byproducts that may contained in the HEU feed. Concentrations of these isotopes in the LEU product in some cases could exceed ASTM specifications for nuclear fuel, if NU, or DU were used. So, the HEU downblending generally cannot contribute to the waste management problem posed by the existing large stockpiles of depleted uranium. A major downblending undertaking called the Megatons to Megawatts Program converts ex-Soviet weapons-grade HEU to fuel for U.S. commercial power reactors. From 1995 through mid-2005, 250 metric tons of high-enriched uranium (enough for 10,000 warheads) was recycled into low-enriched-uranium. The goal is to recycle 500 metric tons by 2013.
Global enrichment facilitiesThe following countries are known to operate enrichment facilities: Argentina, Brazil, China, France, Germany, India, Iran, Japan, the Netherlands, Pakistan, Russia, the United Kingdom and the United States. Israel and North Korea are also suspected of having enrichment programs. Belgium, Iran, Italy and Spain hold an investment interest in the French Eurodif enrichment plant, with Iran's holding entitling it to 10% of the enriched uranium output. Countries that had enrichment programs in the past include Libya and South Africa, although Libya's facility was never operational.[3] Australia has announced its intention to pursue commercial enrichment, and is actively researching laser enrichment.[4] References
See also
Categories: Uranium | Nuclear fuels |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Enriched_uranium". A list of authors is available in Wikipedia. |





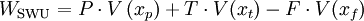
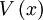 is the value function, defined as
is the value function, defined as