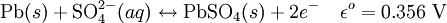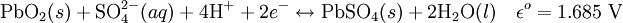To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Lead-acid battery
Lead-acid batteries, invented in 1859 by French physicist Gaston Planté, are the oldest type of rechargeable battery. Despite having the second lowest energy-to-weight ratio (next to the nickel-iron battery) and a correspondingly low energy-to-volume ratio, their ability to supply high surge currents means that the cells maintain a relatively large power-to-weight ratio. These features, along with their low cost, makes them attractive for use in cars, as they can provide the high current required by automobile starter motors. They are also used in vehicles such as forklifts, in which the low energy-to-weight ratio may in fact be considered a benefit since the battery can be used as a counterweight. Large arrays of lead-acid cells are used as standby power sources for telecommunications facilities, generating stations, and computer data centers. They are also used to power the electric motors in diesel-electric (conventional) submarines. Product highlight
ElectrochemistryEach cell contains (in the charged state) electrodes of lead metal (Pb) and lead (IV) oxide (PbO2) in an electrolyte of about 37% w/w (5.99 Molar) sulfuric acid (H2SO4). In the discharged state both electrodes turn into lead(II) sulfate (PbSO4) and the electrolyte loses its dissolved sulfuric acid and becomes primarily water. Due to the freezing-point depression of water, as the battery discharges and the concentration of sulfuric acid decreases, the electrolyte is more likely to freeze. The chemical reactions are (charged to discharged): Cathode (reduction):
Because of the open cells with liquid electrolyte in most lead-acid batteries, overcharging with excessive charging voltages will generate oxygen and hydrogen gas by electrolysis of water, forming an explosive mix. This should be avoided. Caution must also be observed because of the extremely corrosive nature of sulfuric acid. Practical cells are usually not made with pure lead but have small amounts of antimony, tin, or calcium alloyed in the plate material. The following are general voltage ranges for six-cell lead-acid batteries:
Because the electrolyte takes part in the charge-discharge reaction, this battery has one major advantage over other chemistries. It is relatively simple to determine the state of charge by merely measuring the specific gravity (S.G.) of the electrolyte, the S.G. falling as the battery discharges. Some battery designs, such as those used in electronic flash units, have a simple hydrometer built in using coloured floating balls of differing density. When used in diesel-electric submarines, the S.G. was regularly measured and written on a blackboard in the control room to apprise the commander as to how much underwater endurance the boat had remaining. Construction of batteryPlatesThe principle of the lead acid cell can be demonstrated with simple sheet lead plates for the two electrodes. However such a construction would only produce around an amp for roughly postcard sized plates, and it would not produce such a current for more than a few minutes. Gaston Planté realised that a plate construction was required that gave a much larger effective surface area. Planté's method of producing the plates has been largely unchanged and is still used in stationary applications. The Faure pasted-plate construction is typical of automotive batteries. Each plate consists of a rectangular lead grid alloyed with antimony or calcium to improve the mechanical characteristics. The holes of the grid are filled with a mixture of red lead and 33% dilute sulphuric acid (Different manufacturers have modified the mixture). The paste is pressed into the holes in the plates which are slightly tapered on both sides to assist in retention of the paste. This porous paste allows the acid to react with the lead inside the plate, increasing the surface area many fold. At this stage the positive and negative plates are similar, however expanders and additives vary their internal chemistry to assist in operation when in use. Once dry, the plates are then stacked together with suitable separators and inserted in the battery container. An odd number of plates is usually used, with one more negative plate than positive. Each alternate plate is connected together. After the acid has been added to the cell, the cell is given its first forming charge. The positive plates gradually turn the chocolate brown colour of lead dioxide, and the negative turn the slate gray of 'spongy' lead. Such a cell is ready to be used. One of the problems with the plates in a lead-acid battery is that the plates change size as the battery charges and discharges, the plates increasing in size as the active material absorbs sulphate from the acid during discharge, and decreasing as they give up the sulphate during charging. This causes the plates to gradually shed the paste during their life. It is important that there is plenty of room underneath the plates to catch this shed material. If this material reaches the plates a shorted cell will occur. SeparatorsSeparators are used between the positive and negative plates of a lead acid battery to prevent short circuit through physical contact, mostly through dendrites (‘treeing’), but also through shedding of the active material. Separators obstruct the flow of ions between the plates and increase the internal resistance of the cell. Various materials have been used to make separators:
An effective separator must possess a number of mechanical properties; applicable considerations include permeability, porosity, pore size distribution, specific surface area, mechanical design and strength, electrical resistance, ionic conductivity, and chemical compatibility with the electrolyte. In service, the separator must have good resistance to acid and oxidation. The area of the separator must be a little larger than the area of the plates to prevent material shorting between the plates. The separators must remain stable over the operating temperature range of the battery. In the battery service condition the following reaction can be shown :
Classification of lead acid batteriesBy production technology
By application
Applications
Historically, lead-acid batteries were used to supply the filament (heater) voltage (usually between 2 and 12 volts with 6 V being most common) in vacuum tube (valve) radio receivers in areas where no mains electricity supply was available. Such radios typically used two batteries: a lead-acid "A" battery for the filament voltage and a higher voltage (45 V–120 V) "dry" non-rechargeable "B" battery for the plate (anode) voltage. Lead-acid batteries are generally used in emergency lighting in case of power failure. CyclesStarting batteriesLead acid batteries designed for starting automotive engines are not designed for deep discharge. They have a large number of thin plates designed for maximum surface area, and therefore maximum current output, but which can easily be damaged by deep discharge. Repeated deep discharges will result in capacity loss and ultimately in premature failure, as the electrodes disintegrate due to mechanical stresses that arise from cycling. A common misconception is that starting batteries should always be kept on float charge. In reality, this practice will encourage corrosion in the electrodes and result in premature failure. Starting batteries should be kept open-circuit but charged regularly (at least once every two weeks) to prevent sulfation. Deep cycle batteriesSpecially designed deep-cycle cells are much less susceptible to degradation due to cycling, and are required for applications where the batteries are regularly discharged, such as photovoltaic systems, electric vehicles (forklift, golf cart, electric cars and other) and uninterruptible power supplies. These batteries have thicker plates that can deliver less peak current, but can withstand frequent discharging.[1] Marine/Motorhome batteries, sometimes called "leisure batteries", are something of a compromise between the two, able to be discharged to a greater degree than automotive batteries, but less so than deep cycle batteries. Fast and slow charge and dischargeWhen a battery is charged or discharged, this initially affects only the reacting chemicals, which are at the interface between the electrodes and the electrolyte. With time, these chemicals at the interface, which we will call an "interface charge", spread by diffusion throughout the volume of the active material. If a battery has been completely discharged (e.g. the car lights were left on overnight) and next is given a fast charge for only a few minutes, then during the short charging time it develops only a charge near the interface. After a few hours this interface charge will spread to the volume of the electrode and electrolyte, leading to an interface charge so low that it may be insufficient to start the car.[2] On the other hand, if the battery is given a slow charge, which takes longer, then the battery will become more fully charged, since then the interface charge has time to redistribute to the volume of the electrodes and electrolyte, and yet is replenished by the charger. Similarly, if a battery is subject to a fast discharge (such as starting a car, which is a draw of some 600 amps) for a few minutes, it will appear to go dead. Most likely it has only lost its interface charge; after a wait of a few minutes it should appear to be operative. On the other hand, if a battery is subject to a slow discharge (such as leaving the car lights on, which is a draw of only 6 amps), then when the battery appears to be dead it likely has been completely discharged. Valve regulated lead acid batteriesThe Valve Regulated Lead Acid (VRLA) battery is one of many types of lead-acid batteries, also known as Maintenance Free (MF) battery. In a VRLA battery the hydrogen and oxygen produced in the cells recombine back into water. In this way there is no leakage and the battery is maintenance free. It became popular on motorcycles because its acid is absorbed into the medium which separates the plates, so it cannot spill, and this medium also lends support to the plates which helps them better to withstand vibration. The electrical characteristics of MF batteries differ somewhat from wet-cell lead-acid batteries, and caution should be exercised in charging and discharging them. Exploding batteriesMaintenance-Free batteries (VRLA batteries) rely on valves fitted to each cell. Normally any hydrogen and oxygen produced in the cell will recombine into water, but malfunction or misuse may cause gas to build up inside the cell. If this happens (e.g. by overcharging the cell) the valve is designed to vent the gas and thereby normalize the pressure, resulting in a characteristic acid smell around the battery. However, if the valve fails (e.g. blocked by dirt or debris) a dangerous pressure can build up inside the cell. A slight jolt can make a spark jump between the posts and ignite the gas causing an explosion. The force is sufficient to burst the plastic casing or blow the top off the battery, and can injure anyone in the vicinity and spray acid and casing shrapnel to the immediate environment. As a warning, swelling in the cell walls of the battery will occur when the internal pressure rises. The deformation of the walls varies from cell to cell, and is greater at the ends where the walls are unsupported by other cells. It is surprising how powerful an explosion can be caused in the small air space above the electrolyte. When one cell explodes, it sets off a chain reaction in the rest. Such overpressurized batteries should be isolated and discarded, taking great care using protective personal equipment (goggles, overalls, gloves etc) during the handling. Environmental concernsCurrently attempts are being made to develop alternatives to the lead-acid battery (particularly for automotive use) because of concerns about the environmental consequences of improper disposal of old batteries and of lead smelting operations. Newer technologies such as nickel-metal hydride batteries (with cooling, if necessary), supercapacitors and barium-titanate battery / supercapacitor hybrids are poised to make lead-acid batteries obsolete for many automotive applications. Ni-Mn is already widely used in hybrid vehicles. Lead-acid battery recycling is one of the most successful recycling programs in the world, with over 97% of all battery lead recycled between 1997 and 2001.[3] Effective Lead pollution control system is a necessity for sustainable environment. There is a continuous improvement in battery recycling plants and furnace designs for greater efficiencies. These recycling follow all emission standards for lead smelters. AdditivesMany vendors sell chemical additives (solid compounds as well as liquid solutions) that supposedly reduce sulfate build up and improve battery condition when added to the electrolyte of a vented lead-acid battery. Such treatments are rarely, if ever, effective. Two compounds used for such purposes are Epsom salts and EDTA. Epsom salts reduce the internal resistance in a weak or damaged battery and may allow a small amount of extended life. EDTA can be used to dissolve the sulphate deposits of heavily discharged plates. However, the dissolved material is then no longer available to participate in the normal charge/discharge cycle, so a battery temporarily revived with EDTA should not be expected to have normal life expectancy. Residual EDTA in the lead-acid cell forms organic acids which will accelerate corrosion of the lead plates and internal connectors. Active material (the positive plate lead peroxide and negative plate spongy lead) changes physical form during discharge, resulting in plate growth, distortion of the active material, and shedding of active material. Once the active material has left the plates, it cannot be restored into position by any chemical treatment. Similarly, internal physical problems such as cracked plates, corroded connectors, or damaged separators cannot be restored chemically. Maintenance precautionsOne precaution in workshops that handle large lead-acid batteries is a supply of ammonia solution to squirt on any spilled battery acid, to neutralize it. Surplus ammonia, and water, evaporate off, leaving a deposit of ammonium sulphate. See also
References
|
|||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Lead-acid_battery". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||