To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Lithium-ion battery
Lithium-ion batteries (sometimes abbreviated Li-ion batteries) are a type of rechargeable battery in which a lithium ion moves between the anode and cathode. The lithium ion moves from the anode to the cathode during discharge and from the cathode to the anode when charging. Lithium ion batteries are commonly used in consumer electronics. They are currently one of the most popular types of battery for portable electronics, with one of the best energy-to-weight ratios, no memory effect, and a slow loss of charge when not in use. They can be dangerous if mistreated and unless care is taken their lifespan may be reduced. Although originally intended for consumer electronics, lithium-ion batteries are growing in popularity for defense, automotive, and aerospace applications due to their high energy density. The three primary functional components of a lithium ion battery are the anode, cathode, and electrolyte, for which a variety of materials may be used. Commercially, the most popular material for the anode is graphite, although materials such as TiS2 were originally used.[3] However, the cathode is generally one of three materials: a layered oxide, such as cobalt oxide, a polyanion, such as lithium iron phosphate, or a spinel, such as manganese oxide. Depending on the choice of material for the anode, cathode, and electrolyte, the voltage, capacity, life, and safety of a lithium ion battery can change dramatically. Lithium ion batteries are not to be confused with lithium batteries, the key difference being that lithium batteries have a metallic lithium anode and lithium ion batteries have an anode material into which lithium inserts. Product highlight
HistoryLithium batteries were first proposed by M.S. Whittingham, then at Exxon, in the 1970s. [4] Whittingham used titanium sulfide as the cathode and lithium metal as the anode. Lithium batteries, in which metallic lithium is the anode, posed severe safety issues. As a result, lithium ion-batteries were developed, in which the anode, like the cathode, is also a material into which lithium ions insert. Lithium-ion batteries came into reality once Bell Labs developed a workable graphite anode[5] to provide an alternative to lithium metal, the lithium battery. Following groundbreaking cathode research by a team led by John Goodenough[6] (then at Oxford University, now at the University of Texas, Austin), the first commercial lithium ion battery was released by Sony in 1991. The cells utilized layered oxide chemistry, specifically lithium cobalt oxide. Used in numerous commercial applications these batteries revolutionized consumer electronics. In 1983, Michael Thackeray and coworkers identified manganese spinel as a cathode material.[7] Spinel showed great promise, since it is a low-cost material, has good electronic and lithium ion conductivity, and possess a three dimensional structure, which gives it good structural stability. Although pure manganese spinel shows fade with cycling, this can be overcome with additional chemical modification of the material.[8] Manganese spinel is currently used in commercial cells. [9] In 1989, Arumugam Manthiram and John Goodenough at the University of Texas at Austin showed that cathodes containing polyanions, such as sulfates, show higher voltage than oxides due to the inductive effect of the polyanion.[10] Following this, in 1996, Goodenough and coworkers discovered the electrochemical utility of the olivine material lithium iron phosphate, LiFePO4. It is an important and emerging cathode material for lithium-ion batteries due in part to its enhanced safety compared to other lithium-ion chemistries. Cells containing lithium iron phosphate cathodes have been commercialized by multiple companies, including Phostech, Valence Technology, and A123Systems. ElectrochemistryThe three participants in the electrochemical reactions in a lithium ion battery are the anode, cathode, and electrolyte. Both the anode and cathode are materials into which lithium inserts and extracts. The process of lithium moving into the anode or cathode is refered to as insertion, and the reverse process, in which lithium moves out of the anode or cathode is referred to as extraction. When discharging of the cell, the lithium is extracted from the anode and inserted into the cathode. When charging the cell, the exact reverse process occurs: lithium is extracted from the cathode and inserted into the anode. The anode of a conventional Li-ion cell is made from carbon, the cathode is a metal oxide, and the electrolyte is a lithium salt in an organic solvent.[citation needed] The underlying chemical reaction that allows Li-ion cells to provide electricity is: 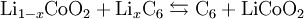 It is important to note that lithium ions themselves are not being oxidized; rather, in a lithium-ion battery the lithium ions are transported to and from the cathode or anode, with the transition metal, Co, in LixCoO2 being oxidized from Co3+ to Co4+ during charging, and reduced from Co4+ to Co3+ during discharge. Cathodes
ElectrolytesLiquid electrolytes in Li-ion batteries consist of solid lithium-salt electrolytes, such as LiPF6, LiBF4, or LiClO4, and organic solvents, such as ether. A liquid electrolyte conducts Li ions, which act as a carrier between the cathode and the anode when a battery passes an electric current through an external circuit. However, solid electrolytes and organic solvents are easily decomposed on anodes during charging, thus preventing battery activation. Nevertheless, when appropriate organic solvents are used for electrolytes, the electrolytes are decomposed and form a solid electrolyte interface at first charge that is electrically insulating and high Li-ion conducting. The interface prevents decomposition of the electrolyte after the second charge. For example, ethylene carbonate is decomposed at a relatively high voltage, 0.7 V vs. Li, and forms a dense and stable interface.[citation needed] See uranium trioxide for some details of how the cathode works. While uranium oxides are not used in commercially made batteries, the way in which uranium oxides can reversibly insert cations is the same as the way in which the cathode in many lithium-ion cells work.[citation needed] Advantages and disadvantagesAdvantagesLithium-ion batteries can be formed into a wide variety of shapes and sizes so as to efficiently fill available space in the devices they power. Li-ion batteries are lighter than other equivalent secondary batteries—often much lighter. The energy is stored in these batteries through the movement of lithium ions. Lithium has the third smallest atomic mass of all the elements giving the battery a substantial saving in weight compared to batteries using much heavier metals. However, the bulk of the electrodes are effectively "housing" for the ions and add weight, and in addition "dead weight" from the electrolyte, current collectors, casing, electronics and conductivity additives reduce the charge per unit mass to little more than that of other rechargeable batteries. A key advantage of using Li-ion chemistry is the high open circuit voltage that can be obtained in comparison to aqueous batteries (such as lead acid, nickel metal hydride and nickel cadmium).[citation needed] Li-ion batteries do not suffer from the memory effect. They also have a low self-discharge rate of approximately 5% per month, compared with over 30% per month in nickel metal hydride batteries and 10% per month in nickel cadmium batteries. According to one manufacturer, Li-ion cells (and, accordingly, "dumb" Li-ion batteries) do not have any self-discharge in the usual meaning of this word.[11] What looks like a self-discharge in these batteries is a permanent loss of capacity, described in more detail below. On the other hand, "smart" Li-ion batteries do self-discharge, due to the small constant drain of the built-in voltage monitoring circuit. This drain is the most important source of self-discharge in these batteries. Disadvantages
A unique drawback of the Li-ion battery is that its life span is dependent upon aging from time of manufacturing (shelf life) regardless of whether it was charged, and not just on the number of charge/discharge cycles. So an older battery will not last as long as a new battery due solely to its age, unlike other batteries. This drawback is not widely publicised.[12] At a 100% charge level, a typical Li-ion laptop battery that is full most of the time at 25 degrees Celsius or 77 degrees Fahrenheit will irreversibly lose approximately 20% capacity per year. However, a battery stored inside a poorly ventilated laptop may be subject to a prolonged exposure to much higher temperatures than 25 °C, which will significantly shorten its life. The capacity loss begins from the time the battery was manufactured, and occurs even when the battery is unused. Different storage temperatures produce different loss results: 6% loss at 0 °C (32 °F), 20% at 25 °C (77 °F), and 35% at 40 °C (104 °F). When stored at 40% - 60% charge level, these figures are reduced to 2%, 4%, 15% at 0, 25 and 40 degrees Celsius respectively. As batteries age, their internal resistance rises. This causes the voltage at the terminals to drop under load, reducing the maximum current that can be drawn from them. Eventually they reach a point at which the battery can no longer operate the equipment it is installed in for an adequate period. High drain applications such as powertools may require the battery to be able to supply a current of 15C - 15 times C - the battery capacity in Ah, whereas MP3 players may only require 0.1C (discharging in 10 hours). With similar technology, the MP3 battery can tolerate a much higher internal resistance, so will have an effective life of many more cycles.[13] Li-ion batteries can even go into a state that is known as deep discharge. At this point, the battery may take a very long time to recharge. For example, a laptop battery that normally charges fully in 3 hours may take up to 42 hours to recharge. Or the deep discharge state may be so severe that the battery will never come back to life. Deep discharging only takes place when products with rechargeable batteries are left unused for extended periods of time (often 2 or more years) or when they are recharged so often that they can no longer hold a charge. This makes Li-ion batteries unsuitable for back-up applications where they may become completely discharged. A stand-alone Li-ion cell must never be discharged below a certain voltage to avoid irreversible damage. Therefore all Li-ion battery systems are equipped with a circuit that shuts down the system when the battery is discharged below the predefined threshold.[11] It should thus be impossible to "deep discharge" the battery in a properly designed system during normal use. This is also one of the reasons Li-ion cells are rarely sold as such to consumers, but only as finished batteries designed to fit a particular system. When the voltage monitoring circuit is built inside the battery (a so-called "smart" battery) rather than the equipment, it continuously draws a small current from the battery even when the battery is not in use; furthermore, the battery must not be stored fully discharged for prolonged periods of time, to avoid damage due to deep discharge. Li-ion batteries are not as durable as nickel metal hydride or nickel-cadmium designs and can be extremely dangerous if mistreated. They are usually more expensive. Li-ion chemistry is not as safe as nickel metal hydride or nickel-cadmium, and a Li-ion cell requires several mandatory safety devices to be built in before it can be considered safe for use outside of a laboratory. These are: shut-down separator (for overtemperature), tear-away tab (for internal pressure), vent (pressure relief), and thermal interrupt (overcurrent/overcharging).[11] The devices take away useful space inside the cells, and add an additional layer of unreliability. Typically, their action is to permanently and irreversibly disable the cell. Approximately 1% of Li-ion batteries are the subject of recalls. [14] (see Controversy). The number of safety features can be compared with that of a nickel metal hydride cell, which only has a hydrogen/oxygen recombination device (preventing damage due to mild overcharging) and a back-up pressure valve.[citation needed] Specifications and design
Because lithium-ion batteries can have a variety of cathode and anode materials, cell specifications, such as the energy density and voltage vary from chemistry to chemistry. Lithium ion batteries with a lithium iron phosphate cathode and graphite anode have a nominal open-circuit voltage of 3.6 V and a typical charging voltage of 4.2 V. The charging procedure is done at constant voltage with current limiting circuitry. This means charging with constant current until a voltage of 4.2 V is reached by the cell and continuing with a constant voltage applied until the current drops close to zero. Typically the charge is terminated at 7% of the initial charge current. In the past, lithium-ion batteries could not be fast-charged and typically needed at least two hours to fully charge. Current generation cells can be fully charged in 45 minutes or less; some Lithium-Ion variants can reach 90% in as little as 10 minutes. [16]
Commercialization of Lithium Ion Batteries
Lithium Cobalt Oxide CathodesLithium ion batteries were first commercialized by Sony in 1991.[17] The cells utilized a lithium cobalt oxide cathode and a graphite anode. Sony and Sanyo are the leading producers of lithium ion batteries.[18][19] A variety of Chinese, Japanese, and South Korean companies produce cells based on the lithium cobalt oxide cathode chemistry.[20] Manganese Spinel CathodesLG Chem, which is the third largest producer of lithium ion batteries, uses the lithium manganese spinel for its cathode. It is working with its subsidiary CPI to commercialize lithium ion batteries containing manganese spinel for HEV applications.[21] Several other companies are also working on manganese spinel, including NEC and Samsung.[22] Lithium Iron Phosphate CathodesThe University of Texas first licensed its patent for lithium iron phosphate cathodes to HydroQuebec.[23] Phostech was later spun-off from Hydroquebec for the sole development of lithium iron phosphate. Valence Technology, located in Austin, TX, is also working on lithium iron phosphate cells. Since March 2005, the Segway Personal Transporter has been shipping with extended-range lithium-ion batteries[24] made by Valence Technology using iron phosphate cathode materials. Segway, Inc chose to build their large-format battery with this cathode material because of its improved safety over metal-oxide materials. In November 2005, A123Systems announced[25] the development of lithium iron phosphate cells [26][27] based on research licensed from MIT. Although MIT and A123 initially claimed to have doped lithium iron phosphate in order to improve the material, it was later shown that this is not the case.[28] The improved performance was due to the presence of an electronically conductive network of carbon surrounding individual lithium iron phosphate particles. Because the original work on this cathode chemistry was done at the University of Texas, the patent licensed by A123 is under litigation. [29] While the battery has a lower energy density that other competing Lithium Ion technologies, a 2 Ahr cell can provide a peak of 70 Amps without damage, and operate at temperatures above 60 degrees C. Their first cell is in production (1Q/2006) and being used in consumer products including DeWalt power tools, aviation products, automotive hybrid systems and PHEV conversions. Titanate AnodesAltairnano,[30] a small firm based in Reno, Nevada, has announced a nano-sized titanate electrode material for lithium-ion batteries. It is claimed the prototype battery has three times the power output of existing batteries and can be fully charged in six minutes. However the energy capacity is about half that of normal li-ion cells. The company also says the battery can handle approximately 20,000 recharging cycles, so durability and battery life are much longer, estimated to be around 20 years or four times longer than regular lithium-ion batteries. The batteries can operate from -50 °C to over 75 °C and will not explode or result in thermal runaway even under severe conditions because they do not contain graphite-coated-metal anode electrode material.[31] The batteries are currently being tested in a new production car made by Phoenix Motorcars which was on display at the 2006 SEMA motorshow. Enerdel, which is jointly owned by Ener1 and Delphi, is working to commercialize cells containing a titanate anode and manganese spinel cathode. [32] Although the cells show excellent thermal properties and cyclability, their low voltage may mitigate commercial success. [33]
Breakthrough ResearchIn April 2006, a group of scientists at MIT announced a process which uses viruses to form nano-sized wires. These can be used to build ultrathin lithium-ion batteries with three times the normal energy density.[34] As of June 2006, researchers in France have created nanostructured battery electrodes with several times the energy capacity, by weight and volume, of conventional electrodes.[35] In December 2007, researchers at Stanford university reported creating a lithium ion battery with ten times the energy density (amount of energy available by weight) through using silicon nanowires deposited on stainless steel as the anode. The battery takes advantage of the fact that silicon can hold large amounts of lithium, and helps alleviate the longstanding problem of cracking by the small size of the wires. [1] To gain a 10fold improvement in energy density, the cathode would need to be improved as well; however, just improving the anode as such could provide a severalfold improvement in energy densitity. The team leader, Yi Cui, expects to be able to commercialize the technology in about five years.[2] Guidelines for prolonging Li-ion battery life
Storage temperature and chargeStoring a Li-ion battery at the correct temperature and charge makes all the difference in maintaining its storage capacity. The following table shows the amount of permanent capacity loss that will occur after storage at a given charge level and temperature.
It is significantly beneficial to avoid storing a lithium-ion battery at full charge. A Li-ion battery stored at 40% charge will last many times longer than one stored at 100% charge, particularly at higher temperatures.[13] If a Li-ion battery is stored with too low a charge, there is a risk of allowing the charge to drop below the battery's low-voltage threshold, resulting in an unrecoverably dead battery. Once the charge has dropped to this level, recharging it can be dangerous. Some batteries therefore feature an internal safety circuit which will prevent charging in this state, and the battery will be for all practical purposes dead.[citation needed] In circumstances where a second Li-ion battery is available for a given device, it is recommended that the unused battery be discharged to 40% and placed in the refrigerator to prolong its shelf life. While the battery can be used or charged immediately, some Li-ion batteries will provide more energy when brought to room temperature. Prolonging Life in Multiple Cells Through Cell BalancingAnalog front ends that balance cells and eliminate mismatches of cells in series or parallel significantly improve battery efficiency and increase the overall pack capacity. As the number of cells and load currents increase, the potential for mismatch also increases. There are two kinds of mismatch in the pack: State-of-Charge (SOC) and capacity/energy (C/E) mismatch. Though the SOC mismatch is more common, each problem limits the pack capacity (mAh) to the capacity of the weakest cell. It is important to recognize that the cell mismatch results more from limitations in process control and inspection than from variations inherent in the Lithium Ion chemistry. The use of cell balancing can improve the performance of series connected Li-ion Cells by addressing both SOC and C/E issues.[38] SOC mismatch can be remedied by balancing the cell during an initial conditioning period and subsequently only during the charge phase. C/E mismatch remedies are more difficult to implement and harder to measure and require balancing during both charge and discharge periods. Cell Balancing Cell balancing is defined as the application of differential currents to individual cells (or combinations of cells) in a series string. Normally, of course, cells in a series string receive identical currents. A battery pack requires additional components and circuitry to achieve cell balancing. However, the use of a fully integrated analog front end for cell balancing[39] reduces the required external components to just balancing resistors. This type of solution eliminates the need for discrete capacitors, diodes and most other resistors to achieve balance. Battery pack cells are balanced when all the cells in the battery pack meet two conditions. 1. If all cells have the same capacity, then they are balanced when they have the same relative State of Charge (SOC.) In this case, the Open Circuit Voltage (OCV) is a good measure of the SOC. If, in an out of balance pack, all cells can be differentially charged to full capacity (balanced), then they will subsequently cycle normally without any additional adjustments. This is mostly a one shot fix. 2. If the cells have different capacities, they are also considered balanced when the SOC is the same. But, since SOC is a relative measure, the absolute amount of capacity for each cell is different. To keep the cells with different capacities at the same SOC, cell balancing must provide differential amounts of current to cells in the series string during both charge and discharge on every cycle. ControversyLithium-ion batteries can rupture, ignite, or explode when exposed to high temperature environments, for example in an area that is prone to prolonged direct sunlight. [40]. Short-circuiting a Li-ion battery can cause it to ignite or explode, and as such, any attempt to open or modify a Li-ion battery's casing or circuitry is dangerous. Li-ion batteries contain safety devices that protect the cells inside from abuse, and, if damaged, can cause the battery to ignite or explode. Contaminants inside the cells can defeat these safety devices. For example, the mid-2006 recall of approximately 10 million Sony batteries used in Dell, Sony, Apple, Lenovo/IBM, Panasonic, Toshiba, Hitachi, Fujitsu and Sharp laptops was stated to be as a consequence of internal contamination with metal particles. Under some circumstances, these can pierce the separator, causing the cell to short, rapidly converting all of the energy in the cell to heat[41]resulting in an exothermic oxidizing reaction (also known as "fire"), increasing the temperature to a few hundred degrees Celsius in a fraction of a second. This causes the neighbouring cells to heat up causing a chain thermal reaction. However, there are problems that go beyond this and so this explanation is not complete. The mid-2006 Sony laptop battery recall was not the first of its kind, however it is the largest to date. During the past decade there have been numerous recalls of lithium-ion batteries in cellular phones and laptops owing to overheating problems. In October 2004, Kyocera Wireless recalled approximately 1 million batteries used in cellular phones, due to counterfeit batteries produced in Kyocera's name.[42] In December 2006, Dell recalled approximately 22,000 batteries from the U.S. market.[43] In March 2007, Lenovo recalled approximately 205,000 9-cell lithium-ion batteries due to an explosion risk. In August 2007, Nokia recalled over 46 million lithium-ion batteries, warning that some of them might overheat and possibly explode.[44] There was an incident in the Philippines involving a Nokia N91, which uses the BL-5C battery.[45] It is possible to replace the lithium cobalt oxide cathode material in li-ion batteries with lithiated metal phosphate cathodes that are not as sensitive to temperature, and so are less prone to explode. This also extends their shelf life. However, currently these 'safer' li-ion batteries are mainly destined for electric cars and other large-capacity battery applications, where the safety issues are more critical. Unfortunately, a problem with these 'safer' li-ion batteries is that lithiated metal phosphate batteries hold only about 75 percent as much [energy].[46] Another option is to use manganese oxide or iron phosphate cathode.[47]
See alsoReferences
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Lithium-ion_battery". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||







