To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Nanowire
A nanowire is a wire of diameter of the order of a nanometer (10−9 meters). Alternatively, nanowires can be defined as structures that have a lateral size constrained to tens of nanometers or less and an unconstrained longitudinal size. At these scales, quantum mechanical effects are important — hence such wires are also known as "quantum wires". Many different types of nanowires exist, including metallic (e.g., Ni, Pt, Au), semiconducting (e.g., Si, InP, GaN, etc.), and insulating (e.g., SiO2,TiO2). Molecular nanowires are composed of repeating molecular units either organic (e.g. DNA) or inorganic (e.g. Mo6S9-xIx). The nanowires could be used, in the near future, to link tiny components into extremely small circuits. Using nanotechnology, such components could be created out of chemical compounds. Product highlight
OverviewTypical nanowires exhibit aspect ratios (length-to-width ratio) of 1000 or more. As such they are often referred to as 1-Dimensional materials. Nanowires have many interesting properties that are not seen in bulk or 3-D materials. This is because electrons in nanowires are quantum confined laterally and thus occupy energy levels that are different from the traditional continuum of energy levels or bands found in bulk materials. Peculiar features of this quantum confinement exhibited by certain nanowires such as carbon nanotubes mainfest themselves in discrete values of the electrical conductance. Such discrete values arise from a quantum mechanical restraint on the number of electrons that can travel through the wire at the nanometer scale. These discrete values are often referred to as the quantum of conductance and are integer values of
They are inverse of the well-known resistance unit h/e², which is roughly equal to 25812.8 ohms, and referred to as the von Klitzing constant RK (after Klaus von Klitzing, the discoverer of exact quantization). Since 1990, a fixed conventional value RK-90 is accepted. Examples of nanowires include inorganic molecular nanowires (Mo6S9-xIx, Li2Mo6Se6), which have a diameter of 0.9 nm, and can be hundreds of micrometers long. Other important examples are based on semiconductors such as InP, Si, GaN, etc., dielectrics (e.g. SiO2,TiO2), or metals (e.g. Ni, Pt). There are many applications where nanowires may become important in electronic, opto-electronic and nanoelectromechanical devices, as additives in advanced composites, for metallic interconnects in nanoscale quantum devices, as field-emittors and as leads for biomolecular nanosensors. Physics of nanowiresProduction of nanowiresNanowires are not observed spontaneously in nature and must be produced in a laboratory. Nanowires can be either suspended, deposited or synthesized from the elements. A suspended nanowire is a wire in vacuum chamber held at the extremities. A deposited nanowire is a wire deposited on a surface of different nature: e.g. it could even be a single strip of metallic atoms over a non-conducting surface. A suspended nanowire can be produced by chemical etching of a bigger wire, or bombarding a bigger wire with some highly energetic particles (atoms or molecules). Another way to produce a suspended nanowire is to indent the tip of an STM in the surface, of a metal near the melting point, and retract it. A common technique for creating a nanowire is the Vapor-Liquid-Solid (VLS) synthesis method. This technique uses as source material either laser ablated particles or a feed gas (such as silane). The source is then exposed to a catalyst. For nanowires, the best catalysts are liquid metal (such as gold) nanoclusters, which can either be purchased in colloidal form and deposited on a substrate or self-assembled from a thin film by dewetting. This process can often produce crystalline nanowires in the case of semiconductor materials. The source enters these nanoclusters and begins to saturate it. Once supersaturation is reached, the source solidifies and grows outward from the nanocluster. The final product's length can be adjusted by simply turning off the source. Compound nanowires with super-lattices of alternating materials can be created by switching sources while still in the growth phase. Inorganic nanowires such as Mo6S9-xIx(which are alternatively viewed as cluster polymers) are synthesised in a single-step vapour phase reaction at elevated temperature. Conductivity of nanowiresThe conductivity of a nanowire is expected to be much less than that of the corresponding bulk material. This is due to a variety of reasons. First, there is scattering from the wire boundaries, when the wire width is below the free electron mean free path of the bulk material. In copper, for example, the mean free path is 40 nm. Nanowires less than 40 nm wide will shorten the mean free path to the wire width. Nanowires also show other peculiar electrical properties due to their size. Unlike carbon nanotubes, whose motion of electrons can fall under the regime of ballistic transport (meaning the electrons can travel freely from one electrode to the other), nanowire conductivity is strongly influenced by edge effects. The edge effects come from atoms that lay at the nanowire surface and are not fully bonded to neighboring atoms like the atoms within the bulk of the nanowire. The unbonded atoms are often a source of defects within the nanowire, and may cause the nanowire to conduct electricity more poorly than the bulk material. As a nanowire shrinks in size, the surface atoms becomes more numerous compared to the atoms within the nanowire, and edge effects become more important. Furthermore the conductivity can undergo a quantization in energy: i.e. the energy of the electrons going through a nanowire can assume only discrete values, multiple of the Von Klitzing constant G = 2e2 / h (where e is the charge of the electron and h is Planck's constant). The conductivity is hence described as the sum of the transport by separate channels of different quantized energy levels. The thinner the wire is, the smaller the number of channels available to the transport of electrons. The conductivity of a nanowire can be studied suspending it between two electrodes. This has been proven by measuring the conductivity of a nanowire while pulling it: while it shrinks, its conductivity decreases in a stepwise fashion and the plateaus correspond to multiples of G. The quantized conductivity is more pronounced in semiconductors like Si or GaAs than in metals, due to lower electron density and lower effective mass. Quantized conductance can be observed in 25 nm wide silicon fins (Tilke et al., 2003), resulting in increased threshold voltage. Structure of nanowiresThe nanowires can show peculiar shapes. Sometimes they can show noncrystalline order, assuming e.g. a pentagonal symmetry or a helicoidal (spiral) shape. Electrons zigzag along pentagonal tubes and spiral along helicoidal tubes. The lack of crystalline order is due to the fact that a nanowire is periodic only in one dimension (along its axis). Hence it can assume any order in the other directions (in plane) if this is energetically favorable. E.g., in some cases nanowires can show a fivefold symmetry, usually not observed in nature, but for clusters of few atoms. The fivefold symmetry is equivalent to the icosahedral symmetry of (small) atomic clusters: the icosahedron is often an energetically favorable shape for cluster of few atoms, but icosahedral ordering is not observed in crystals since it is not possible to stack together icosahedra (repeating infinite copies of them in each direction) and tile the whole space (fill it without holes). Use of nanowiresNanowires still belong to the experimental world of laboratories. However, they may complement or replace carbon nanotubes in some applications. Some early experiments have shown how they can be used to build the next generation of computing devices. To create active electronic elements, the first key step was to chemically dope a semiconductor nanowire. This has already been done to individual nanowires to create p-type and n-type semiconductors. The next step was to find a way to create a p-n junction, one of the simplest electronic devices. This was achieved in two ways. The first way was to physically cross a p-type wire over an n-type wire. The second method involved chemically doping a single wire with different dopants along the length. This method created a p-n junction with only one wire. After p-n junctions were built with nanowires, the next logical step was to build logic gates. By connecting several p-n junctions together, researchers have been able to create the basis of all logic circuits: the AND, OR, and NOT gates have all been built from semiconductor nanowire crossings. It's possible that semiconductor nanowire crossings will be important to the future of digital computing. Though there are other uses for nanowires beyond these, the only ones that actually take advantage of physics in the nanometer regime are electronic. Nanowires are being studied for use as photon ballistic waveguides as interconnects in quantum dot/quantum effect well photon logic arrays. Photons travel inside the tube, electrons travel on the outside shell. When two nanowires acting as photon waveguides cross each other the juncture acts as a quantum dot. Conducting nanowires offer the possibility of connecting molecular-scale entities in a molecular computer. Dispersions of conducing nanowires in different polymers are being investigated for use as transparent electrodes for flexible flat-screen displays. Due to their high Young's moduli their use in mechanically enhancing composites is being investigated. Because nanowires appear in bundles, they may be used as tribological additives to improve friction characteristics and reliability of electronic transducers and actuators. Because of their high aspect ratio, nanowires are also uniquely suited to dielectrophoretic manipulation. References
See also
|
||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Nanowire". A list of authors is available in Wikipedia. |





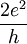 ≈ 12.9 kΩ-1
≈ 12.9 kΩ-1


