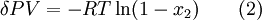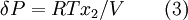To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Osmosis
Osmosis is the spontaneous net movement of water through a semipermeable membrane from a region of low solute concentration to a solution with a high solute concentration, up a solute concentration gradient. It is a physical process in which a solvent moves, without input of energy, across a semi permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations.[1] Osmosis releases energy, and can be made to do work, as when a growing tree-root splits a stone. Net movement of solvent is from the less-concentrated (hypotonic) to the more-concentrated (hypertonic) solution, which tends to reduce the difference in concentrations. This effect can be countered by increasing the pressure of the hypertonic solution, with respect to the hypotonic. The osmotic pressure is defined to be the pressure required to maintain an equilibrium, with no net movement of solvent. Osmotic pressure is a colligative property, meaning that the property depends on the molar concentration of the solute but not on its identity. Osmosis is the result of diffusion across a semi-permeable membrane. Osmosis is important in biological systems as many biological membranes are semipermeable. In general, these membranes are impermeable to organic solutes with large molecules, such as polysaccharides, while permeable to water and small, uncharged solutes. Permeability may depend on solubility properties, charge, or chemistry as well as solute size. Water molecules travel through the plasma cell wall, tonoplast (vacuole) or protoplast in two ways. Either by diffusing across the phospholipid bilayer directly, or via aquaporins (small transmembrane proteins similar to those in facilitated diffusion and in creating ion channels). Osmosis provides the primary means by which water is transported into and out of cells. The turgor pressure of a cell is largely maintained by osmosis, across the cell membrane, between the cell interior and its relatively hypotonic environment. Product highlight
Basic explanationConsider a semi-permeable membrane, such as visking tubing, with apertures big enough to allow water (solvent) molecules, but not larger solute molecules, to pass through. When this membrane is immersed in liquid it is constantly hit by molecules of the liquid, in motion due to their thermal kinetic energy. In this respect solute and solvent molecules are indistinguishable. At a molecular scale, every time a molecule hits the membrane it has a defined likelihood of passing through. Here, there is a difference: for water molecules this probability is non-zero; for solute molecules it is zero. Suppose the membrane is in a volume of pure water. In this case, since the circumstances on both sides of the membrane are equivalent, water molecules pass in each direction at the same rate; there is no net flow of water through the membrane. If there is a solution on one side, and pure water on the other, the membrane is still hit by molecules from both sides at the same rate. However, some of the molecules hitting the membrane from the solution side will be solute molecules, and these will not pass through the membrane. So water molecules pass through the membrane from this side at a slower rate. This will result in a net flow of water to the side with the solution. Assuming the membrane does not break, this net flow will slow and finally stop as the pressure on the solution side becomes such that the movement in each direction is equal: dynamic equilibrium. This could either be due to the water potential on both sides of the membrane being the same, or due to osmosis being inhibited by factors such as pressure potential or Osmotic pressure. Osmosis can also be explained via the notion of entropy, from statistical mechanics. As above, suppose a permeable membrane separates equal amounts of pure solvent and a solution. Since a solution possesses more entropy than pure solvent, the second law of thermodynamics states that solvent molecules will flow into the solution until the entropy of the combined system is maximized. Notice that, as this happens, the solvent loses entropy while the solution gains entropy. Equilibrium, hence maximum entropy, is achieved when the entropy gradient becomes zero. Examples of osmosisOsmotic pressure is the main cause of support in many plants. The osmotic entry of water raises the turgor pressure exerted against the cell wall, until it equals the osmotic pressure, creating a steady state. When a plant cell is placed in a hypertonic solution, the water in the cells moves to an area higher in solute concentration, and the cell shrinks and so becomes flaccid. (This means the cell has become plasmolysed - the cell membrane has completely left the cell wall due to lack of water pressure on it; the opposite of turgid.) Also, osmosis is responsible for the ability of plant roots to suck up water from the soil. Since there are many fine roots, they have a large surface area, water enters the roots by osmosis. Osmosis can also be seen very effectively when potato slices are added to a high concentration of salt solution. The water from inside the potato moves to the salt solution, causing the potato to shrink and to lose its 'turgor pressure'. The more concentrated the salt solution, the bigger the difference in size and weight of the potato slice. In unusual environments, osmosis can be very harmful to organisms. For example, freshwater and saltwater aquarium fish placed in water of a different salinity than that they are adapted to will die quickly, and in the case of saltwater fish, rather dramatically. Another example of a harmful osmotic effect is the use of table salt to kill leeches and slugs. Suppose an animal or a plant cell is placed in a solution of sugar or salt in water.
Chemical gardens demonstrate the effect of osmosis in inorganic chemistry. Osmotic pressureAs mentioned before, osmosis may be opposed by increasing the pressure in the region of high solute concentration with respect to that in the low solute concentration region. The force per unit area, or pressure, required to prevent the passage of water through a selectively-permeable membrane and into a solution of greater concentration is equivalent to the osmotic pressure of the solution, or turgor. Osmotic pressure is a colligative property, meaning that the property depends on the concentration of the solute but not on its identity. Increasing the pressure increases the chemical potential of the system in proportion to the molar volume (δμ = δPV). Therefore, osmosis stops when the increase in potential due to pressure equals the potential decrease from Equation 1, i.e.:
Where δP is the osmotic pressure and V is the molar volume of the solvent.
Osmotic gradientThe osmotic gradient is the difference in concentration between two solutions on either side of a semipermeable membrane, and is used to tell the difference in percentages of the concentration of a specific particle dissolved in a solution. Usually the osmotic gradient is used while comparing solutions that have a semipermeable membrane between them allowing water to diffuse between the two solutions, toward the hypertonic solution(the solution with the higher concentration). Eventually, the force of the column of water on the hypertonic side of the semipermeable membrane will equal the force of diffusion on the hypotonic (the side with a lesser concentration) side, creating equilibrium. When equilibrium is reached, water continues to flow, but it flows both ways in equal amounts as well as force, therefore stabilizing the solution. Reverse osmosisForward osmosisOsmosis may be used directly to achieve separation of water from a "feed" solution containing unwanted solutes. A "draw" solution of higher osmotic pressure than the feed solution is used to induce a net flow of water through a semi-permeable membrane, such that the feed solution becomes concentrated as the draw solution becomes dilute. The diluted draw solution may then be used directly (as with an ingestible solute like glucose), or sent to a secondary separation process for the removal of the draw solute. This secondary separation can be more efficient than a reverse osmosis process would be alone, depending on the draw solute used and the feedwater treated. Forward osmosis is an area of ongoing research, focusing on applications in desalination, water purification, water treatment, food processing, etc. See alsoWikibooks' [[wikibooks:|]] has more about this subject:
School science/Osmosis demonstration
ReferencesCategories: Diffusion | Water technology |
|||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Osmosis". A list of authors is available in Wikipedia. |