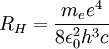To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Rydberg constant
The Rydberg constant, named after physicist Johannes Rydberg, is a physical constant that appears in the Rydberg formula. It was discovered when measuring the spectrum of hydrogen, and builds upon results from Anders Jonas Ångström and Johann Balmer. The "infinite" Rydberg constant is often simply called the "Rydberg constant" and is essentially the (cyclical) wavenumber of the photon emitted when a Hydrogen atom decays from n = infinity (unbound electron and proton) directly into the ground state, n = 1. Thus it also represents the minimum wavenumber a single photon must have in order to completely free the electron of a hydrogen atom in the ground state. The Rydberg constant is one of the most well-determined physical constants, with a relative experimental uncertainty of less than 7 parts per trillion. The ability to measure it directly to such a high precision confirms the proportions of the values of the other physical constants that define it, and can thus be used to stringently test physical theories such as quantum electrodynamics. Each chemical element has its own Rydberg constant. For all Hydrogen-like atoms (atoms with a single electron in their outermost orbit) the Rydberg constant
The "infinity" Rydberg constant is (according to 2002 CODATA results):
This constant is often used in atomic physics in the form of an energy: Product highlight
Alternate expressionsThe Rydberg constant can also be expressed as the following equations. and where
Rydberg constant for hydrogenSubstituting the 2002 CODATA value for the electron-proton mass ratio, Substituting Where
Derivation of Rydberg constantThe Rydberg constant for hydrogen can be derived using Bohr's condition, centripetal force, electric force, and electric potential energy of an electron in orbit around a proton (corresponding to the case for the hydrogen atom).
To begin, we take Bohr's primary condition and solve it in terms of the electron's permitted orbital velocity v:
Since the electric force attracting the electron to the nucleus is the (centripetal) force driving the electron into a circular orbit around the proton, we can set Fcentripetal = Felectric to obtain
Substitute our previous expression for the electron orbital velocity
This value of r supposedly represents the only allowed values for the orbital radius of an electron in orbit around a proton assuming the Bohr condition holds for the wave nature of the electron. If we now substitute r into the expression for the electric potential energy of an electron some distance from a proton and we get
Therefore a change in energy in an electron changing from one value of n to another is
We simply change the units to wavelength
where
We have therefore found the Rydberg constant for Hydrogen to be
See alsoReferencesMathworld |
|||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Rydberg_constant". A list of authors is available in Wikipedia. |


 can be derived from the "infinity" Rydberg constant, as follows:
can be derived from the "infinity" Rydberg constant, as follows:
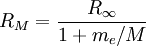
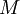 is the mass of its
is the mass of its 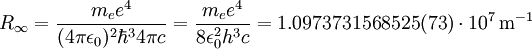
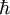 is the reduced Planck's constant,
is the reduced Planck's constant,
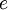 is the elementary charge,
is the elementary charge,
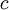 is the
is the 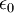 is the permittivity of free space.
is the permittivity of free space.
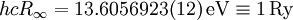



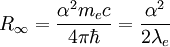
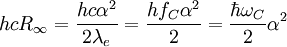
 is Planck's constant,
is Planck's constant,
 is the fine-structure constant,
is the fine-structure constant,
 is the
is the 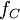 is the Compton frequency of the electron,
is the Compton frequency of the electron,
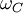 is the Compton angular frequency of the electron.
is the Compton angular frequency of the electron.
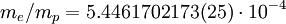 , into the general formula for the Rydberg constant for any Hydrogen-like element
, into the general formula for the Rydberg constant for any Hydrogen-like element 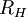 .
.
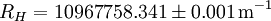
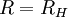 into the
into the 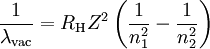
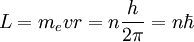
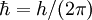 .
.
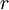 is the radius of the electron's orbit
is the radius of the electron's orbit
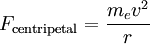
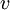 is the electron's velocity
is the electron's velocity
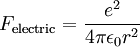
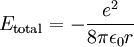
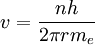
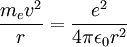
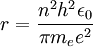
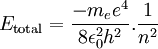
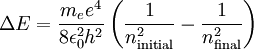
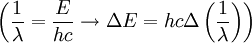 and we get
and we get
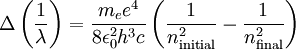
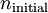 and
and 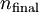 being the electron shell number of the hydrogen atom
being the electron shell number of the hydrogen atom