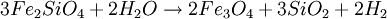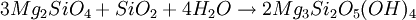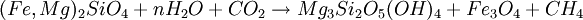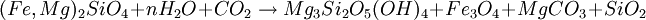To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
SerpentiniteSerpentinite is a rock comprised of one or more serpentine minerals. Minerals in this group are formed by serpentinization, a hydration and metamorphic transformation of ultramafic rock from the Earth's mantle. The alteration is particularly important at the sea floor at tectonic plate boundaries. It is the state rock of California, USA. Product highlight
FormationSerpentinization is a geological low-temperature and metamorphic process involving heat and water in which low-silica mafic and ultramafic rocks are oxidized and hydrolyzed with water into serpentinite. Peridotite, including dunite, at and near the seafloor and in mountain belts is converted to serpentine, brucite, magnetite, and other minerals -- some rare, such as awaruite (Ni3Fe), and even native iron. In the process large amounts of water are absorbed into the rock increasing the volume and destroying the structure. The density changes from 3.3 to 2.7 g/cm3 with a concurrent volume increase of about 40%. The reaction is exothermic and large amounts of heat energy are produced in the process. Rock temperatures can be raised by about 260 oC, providing an energy source for formation of non-volcanic hydrothermal vents. The magnetite-forming chemical reactions produce hydrogen gas. Sulfates and carbonates are reduced and form methane and hydrogen sulfide. The hydrogen, methane, and hydrogen sulfide provide energy sources for deep sea chemotroph microorganisms. Serpentinite reactionsSerpentinite is formed from olivine via several reactions, some of which are complementary. Olivine is a solid solution between the magnesium-endmember forsterite and the iron-endmember fayalite. Serpentinite reactions 1a and 1b, below, exchange silica between forsterite and fayalite to form serpentine-group minerals and magnetite. These are highly exothermic reactions. Reaction 1a: Reaction 1b: Other possibilities include the reaction of olivine plus water to yield serpentine plus Mg(OH)2 (brucite), and reactions in which magnesium and silica are transported out of the serpentinizing volume. A similar suite of reactions involves pyroxene-group minerals, though less readily and with complication of the additional end-products due to the wider compositions of pyroxene and pyroxene-olivine mixes. Talc and magnesian chlorite are possible products, together with the serpentine minerals antigorite, lizardite, and chrysotile. The final mineralogy depends both on rock and fluid compositions, temperature, and pressure. Antigorite forms in reactions at temperatures that can exceeed 600°C during metamorphism, and it is the serpentine-group mineral stable at the highest temperatures. Lizardite and chrysotile can form at low temperatures very near the Earth's surface. Fluids involved in serpentinite formation commonly are highly reactive and may transport calcium and other elements into surrounding rocks; fluid reaction with these rocks may create metasomatic reaction zones enriched in calcium and called rodingites. In the presence of carbon dioxide, however, serpentinitization may form either magnesite (MgCO3) or generate methane (CH4). It is thought that some hydrocarbon gases may be produced by serpentinite reactions within the oceanic crust, and the serpentinite reaction is a key argument for the theory of abiogenic petroleum origin. Reaction 2a:
or, in balanced form: 18Mg2SiO4 + 6Fe2SiO4 + 26H2O + CO2 → 12Mg3Si2O5(OH)4 + 4Fe3O4 + CH4 Reaction 2b:
Reaction 2a is favored if the serpentinite is Mg-poor or if there isn't enough carbon dioxide to promote talc formation. Reaction 2b is favored in highly magnesian compositions and low partial pressure of carbon dioxide. (Something is missing from Reaction 2b because Fe is oxidized and nothing gets reduced.) The degree to which a mass of ultramafic rock undergoes serpentinisation depends on the starting rock composition and on whether or not fluids transport calcium, magnesium and other elements away during the process. If an olivine composition contains sufficient fayalite, then olivine plus water can completely metamorphose to serpentine and magnetite in a closed system. In most ultramafic rocks formed in the Earth's mantle, however, the olivine is about 90% forsterite endmember, and for that olivine to react completely to serpentine, magnesium must be transported out of the reacting volume. Serpentinitization of a mass of peridotite usually destroys all previous textural evidence because the serpentine minerals are weak and behave in a very ductile fashion. However, some masses of serpentinite are less severely deformed, as evidenced by the apparent preservation of textures inherited from the peridotite, and the serpentinites may have behaved in a rigid fashion. Carbon sequestrationSerpentinite has been proposed as an efficient reagent for carbon sequestration using the magnesite reaction, above, or a variation where serpentine is reacted with carbon dioxde and hydrogen to form magnesite, magnetite, silica. The ideal composition of olivine or serpentinite for this process is thus highly magnesian, to favor production of magnesite and the fixation of carbon. Bleistein or OfensteinA lamelled variety of serpentinite is found in South Tyrol and is locally called Bleistein (Leadstone) or Ofenstein (Ovenstone). It was used primarily for building wood burning indoor heating stoves as it has the capacity of storing and dispersing heat very well along the veins. A New York educational documentary film production company [1] is releasing a film that describes the quarrying of this stone and its use in the construction of stoves. See also
References
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Serpentinite". A list of authors is available in Wikipedia. |