To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Thermal ionizationIn thermal ionization, also referred to as surface ionization, chemically-purified material loaded onto a filament which is then heated to cause some of the material to be ionized as it boils off the hot filament. Filaments are generally flat pieces of metal around 1-2mm wide, 0.1mm thick, bent into an upside-down U shape and welded to steel posts that supply a current. Product highlight
Saha-Langmuir equationThe likelihood of ionisation is a function of the filament temperature, the work function of the filament substrate and the ionization energy of the element. This is summarised in the Saha-Langmuir equation:[1]
Thermal ionization mass spectrometryOne application of thermal ionization is thermal ionization mass spectrometry (TIMS). The ions being produced at the filament are directed into a mass spectrometer to analyze the elements or isotopes present in the sample.[2] See also
References
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Thermal_ionization". A list of authors is available in Wikipedia. |





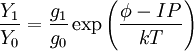
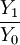 = ion to neutral ratio
= ion to neutral ratio
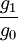 = statistical weights of ion and neutral states
= statistical weights of ion and neutral states


