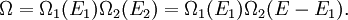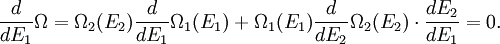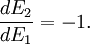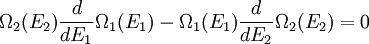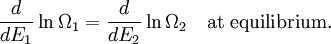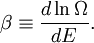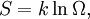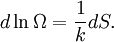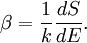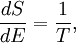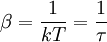To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Thermodynamic betaIn statistical mechanics, the thermodynamic beta is a numerical quantity related to the thermodynamic temperature of a system. The thermodynamic beta can be viewed as a connection between the statistical interpretation of a physical system and thermodynamics. Product highlight
DetailsStatistical interpretationFrom the statistical point of view, β is a numerical quantity relating two macroscopic systems in equilibrium. The exact formulation is as follows. Consider two systems, 1 and 2, in thermal contact, with respective energies E1 and E2. We assume E1 + E2 = some constant E. The number of microstates of each system will be denoted by Ω1 and Ω2. Under our assumptions Ωi depends only on Ei. Thus the number of microstates for the combined system is We will derive β from the following fundamental assumption:
(In other words, the system naturally seeks the maximum number of microstates.) Therefore, at equilibrium, But E1 + E2 = E implies So i.e. The above relation motivates the definition of β: Connection with thermodynamic viewOn the other hand, when two systems are in equilibrium, they have the same thermodynamic temperature T. Thus intuitively one would expect that β be related to T in some way. This link is provided by the formula where k is the Boltzmann constant. So Substituting into the definition of β gives Comparing with the thermodynamic formula we have where τ is sometimes called the fundamental temperature of the system with units of energy. See alsoCategories: Thermodynamics | Units of temperature |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Thermodynamic_beta". A list of authors is available in Wikipedia. |