To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Weak acid
A weak acid is an acid that does not ionise in a solution to a significant extent; that is, if the acid was represented by the general formula HA, then in aqueous solution a significant amount of undissociated HA still remains. Weak acids in water dissociate as Product highlight
The equilibrium concentrations of reactants and products are related by the Acidity constant expression, (Ka):
The greater the value of Ka, the more the formation of H+ is favored, and the lower the pH of the solution. The Ka of weak acids varies between 1.8×10-16 and 55.5. Acids with a Ka less than 1.8×10-16 are weaker acids than water. Acids with a Ka of greater than 55.5 are strong acids and almost totally dissociate when dissolved in water. The vast majority of acids are weak acids. Organic acids are a large subset of weak acids. Common household weak organic acids include acetic acid found in vinegar, and citric acid found in lemons; weak mineral acids include boric acid used as an antiseptic and eyewash and phosphoric acid that appears in many soft drinks. See also |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Weak_acid". A list of authors is available in Wikipedia. |





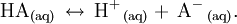
![\mathrm{ K_a\, =\, \frac {[H^+\,][A^-\,]}{[HA]} }](images/math/e/1/1/e1160cb29e6e647fa04612aee2af4f35.png)


