Pfizer and Bristol-Myers Squibb Finalize Agreement for Worldwide Collaboration on Metabolic Disorders Program
Companies Will Jointly Conduct Phase III Development and Commercialization of DGAT-1 Inhibitor Compounds
Advertisement
Pfizer Inc and Bristol-Myers Squibb Company announced that they have finalized a definitive agreement for the worldwide collaboration to research, develop and commercialize DGAT-1 inhibitors. Pfizer's DGAT-1 discovery program includes advanced pre-clinical compounds with potential applications for the treatment of metabolic disorders, including obesity and diabetes. The program also includes DGAT-1 inhibitors in-licensed by Pfizer from Bayer Pharmaceuticals Corporation in June 2006, including a pre-clinical compound (known as PF-04415060 or BAY 74-4113) originally discovered by Bayer.
Under terms of the agreement, Pfizer will be responsible for all research and early-stage development activities for the metabolic disorders program, and the companies will jointly conduct Phase III development and commercialization activities.
Triglycerides are the principal component of fat, which is the major repository for storage of metabolic energy in the body. DGAT-1 (diacylglycerol acyl transferase-1) is an enzyme critical to the creation of triglycerides and fat storage. Overweight and obese individuals have significantly greater triglyceride levels, making them more prone to diabetes and its associated metabolic complications. In studies of obese animals, DGAT-1 inhibitors have been shown to induce weight loss and improve glucose tolerance and lipid levels. These observations suggest DGAT-1 inhibitors may have the potential to treat obesity, diabetes and dyslipidemia.
Most read news
Organizations
Other news from the department business & finance

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Graphene is on track to deliver on its promises
Schrödinger cats snatch many-particle quantum states
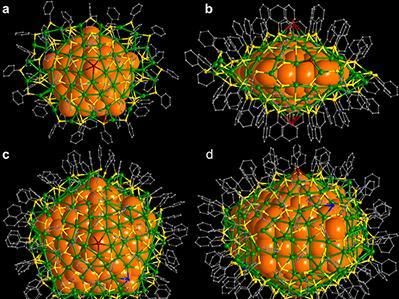
Researchers synthesize atomically precise diamond-shaped nanoclusters of silver
A micro-supercapacitor with unmatched energy storage performance