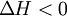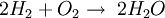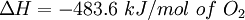To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Exothermic reactionThe total energy in bond breaking is less than the total energy released in bond making. In chemistry, an exothermic reaction is one that releases heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation: Product highlight
OverviewIn an exothermic reaction, the total energy absorbed in bond breaking is less than the total energy released in bond making. In other words, the energy needed for the reaction to occur is less than the total energy provided. As a result of this, the extra energy is released, usually in the form of heat. When using a calorimeter, the change in heat of the calorimeter is equal to to the opposite of the change in heat of the system. This means that when the medium in which the reaction is taking place gains heat, the reaction is exothermic. The absolute amount of energy in a chemical system is extremely difficult to measure or calculate. The enthalpy change, ΔH, of a chemical reaction is much easier to measure and calculate. A bomb calorimeter is very suitable for measuring the energy change, ΔH, of a combustion reaction. Measured and calculated ΔH values are related to bond energies by:
by definition the enthalpy change has a negative value: For an exothermic reaction, this gives a negative value for ΔH, since a larger value (the energy released in the reaction) is subtracted from a smaller value (the energy used for the reaction). For example, when hydrogen burns: Examples of exothermic reactions
Key points
See also
Categories: Thermodynamics | Chemical reactions |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Exothermic_reaction". A list of authors is available in Wikipedia. |


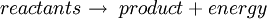



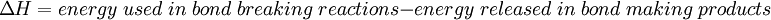 .
.