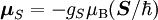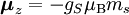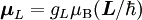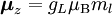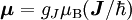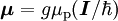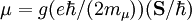To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
G-factorTemplate:DISPLAYTITLE:g-factor
A g-factor (also called g value or dimensionless magnetic moment) is a dimensionless quantity which characterizes the magnetic moment and gyromagnetic ratio of a particle or nucleus. It is essentially a proportionality constant that relates the observed magnetic moment μ of a particle to the appropriate angular momentum quantum number and the fundamental quantum unit of magnetism, the Bohr magneton. Additional recommended knowledge
Electron g-factorsThere are three magnetic moments associated with an electron: One from its spin angular momentum, one from its orbital angular momentum, and one from its total angular momentum (the quantum-mechanical sum of those two components). Corresponding to these three moments are three different g-factors: Electron spin g-factorThe most famous of these is the electron spin g-factor, gS (more often called simply the electron g-factor, ge), defined by where μS is the total magnetic moment resulting from the spin of an electron, S is the magnitude of its spin angular momentum, and μB is the Bohr magneton. The z-component of the magnetic moment then becomes The value gS is roughly equal to two, and is known to extraordinary accuracy.[1][2] The reason it is not precisely two is explained by quantum electrodynamics.[3] Electron orbital g-factorSecondly, the electron orbital g-factor, gL, is defined by where μL is the total magnetic moment resulting from the orbital angular momentum of an electron, L is the magnitude of its orbital angular momentum, and μB is the Bohr magneton. The value of gL is exactly equal to one, by a quantum-mechanical argument analogous to the derivation of the classical magnetogyric ratio. For an electron in an orbital with a magnetic quantum number ml, the z-component of the orbital angular momentum is which, since gL = 1, is just μBml Landé g-factorThirdly, the Landé g-factor, gJ, is defined by where μ is the total magnetic moment resulting from both spin and orbital angular momentum of an electron, J = L+S is its total angular momentum, and μB is the Bohr magneton. The value of gJ is related to gL and gS by a quantum-mechanical argument; see the article Landé g-factor. Nucleon and Nucleus g-factorsProtons, neutrons, and many nuclei have spin and magnetic moments, and therefore associated g-factors. The formula conventionally used is where μ is the magnetic moment resulting from the nuclear spin, I is the nuclear spin angular momentum, and μp is the nuclear magneton. Muon g-factorThe muon, like the electron has a g-factor from its spin, given by the equation where μ is the magnetic moment resulting from the muon’s spin, S is the spin angular momentum, and mμ is the muon mass.
The muon g-factor can be affected by physics beyond the Standard Model, so has been measured very precisely, in particular at the Brookhaven National Laboratory. As of November 2006, the experimentally measured value is 2.0023318416 with an uncertainy of 0.0000000013, compared to the theoretical prediction of 2.0023318361 with an uncertainty of 0.0000000010[4]. This is a difference of 3.4 standard deviations, suggesting beyond-the-Standard-Model physics may be having an effect. Measured g-factor Values
It should be noted that the electron g-factor is one of the most precisely measured values in physics, with its uncertainty beginning at the twelfth decimal place. Notes and references
See also
|
||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "G-factor". A list of authors is available in Wikipedia. |