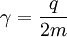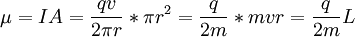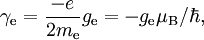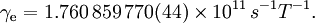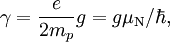To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Gyromagnetic ratioIn physics, the gyromagnetic ratio (also sometimes known as the magnetogyric ratio in other disciplines) of a particle or system is the ratio of its magnetic dipole moment to its angular momentum, and it is often denoted by the symbol γ, gamma. Its SI units are radian per second per tesla (s-1T -1) or, equivalently, coulomb per kilogram (C/kg). Product highlight
Gyromagnetic ratio and Larmor precessionAny free system with a constant gyromagnetic ratio, such as a rigid system of charges, a nucleus, or an electron, when placed in an external magnetic field B (measured in teslas) that is not aligned with its magnetic moment, will precess at a frequency f (measured in hertz), that is proportional to the external field:
For this reason, values of γ/(2π), in units of hertz per tesla (Hz/T), are often quoted instead of γ. This relationship also explains an apparent contradiction between the two equivalent terms, gyromagnetic ratio versus magnetogyric ratio: whereas it is a ratio of a magnetic property (i.e. dipole moment) to a gyric (rotational, from Greek: γύρος, "turn") property (i.e. angular momentum), it is also, at the same time, a ratio between the angular precession frequency (another gyric property) ω = 2πf and the magnetic field. Gyromagnetic ratio for a classical rotating bodyConsider a charged body rotating about an axis of symmetry. According to the laws of classical physics, it has both a magnetic dipole moment and an angular momentum on account of its rotation. It can be shown that as long as its charge and mass are distributed identically (e.g., both distributed uniformly), its gyromagnetic ratio is where q is its charge and m is its mass. The derivation of this relation is as follows: It suffices to demonstrate this for an infinitesimally narrow circular ring within the body, as the general result follows from an integration. Suppose the ring has radius r, area A = πr2, mass m, charge q, and angular momentum L=mvr. Then the magnitude of the magnetic dipole moment is as desired. Gyromagnetic ratio for an isolated electronAn isolated electron has an angular momentum and a magnetic moment resulting from its spin. While an electron's spin is sometimes visualized as a literal rotation about an axis, it is in fact a fundamentally different, quantum-mechanical phenomenon[1] with no true analogue in classical physics. Consequently, there is no reason to expect the above classical relation to hold. In fact it does not, giving the wrong result by a dimensionless factor called the electron g-factor, denoted ge (or just g when there is no risk of confusion): where μB is the Bohr magneton. The electron g-factor ge is a bit more than two, and has been measured to twelve decimal places:[2]
The electron gyromagnetic ratio is given by NIST[3] as Gyromagnetic ratio for a nucleusProtons, neutrons, and many nuclei carry nuclear spin, which gives rise to a gyromagnetic ratio as above. The ratio is conventionally written in terms of the proton mass and charge, even for neutrons and for other nuclei, for the sake of simplicity and consistency. The formula is: where μN is the nuclear magneton, and g is the g-factor of the nucleon or nucleus in question. The gyromagnetic ratio of a nucleus is particularly important because of the role it plays in Nuclear Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI). These procedures rely on the fact that nuclear spins precess in a magnetic field at a rate called the Larmor frequency, which is simply the product of the gyromagnetic ratio with the magnetic field strength. Approximate values for some common nuclei are given in the Table below.[4]
See alsoReferences
|
|||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Gyromagnetic_ratio". A list of authors is available in Wikipedia. |





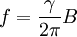 .
.