Snowflakes become square with a little help from graphene
water is one of the most familiar and abundant substances on Earth. It exists in many forms, as liquid, vapour and as many as 15 crystal structures of ice, with the commonly found hexagonal ice being singlehandedly responsible for the fascinating variety of snowflakes.
Less noticeable but equally ubiquitous is water at interfaces and confined in microscopic pores. In fact, a few monolayers of water cover every surface around us, even in driest deserts, and fill in every single microscopic crack, for example, those present in rocks.
Yet, very little is known about the structure and behaviour of such microscopic water, especially when it is hidden from the view, in capillaries deep inside a bulk material.
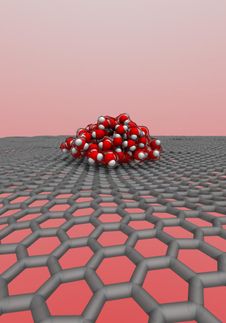
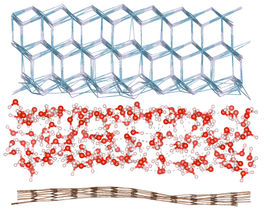
An international team of researchers from The University of Manchester, the University of Ulm in Germany and the University of Science and Technology of China have created a transparent nanoscale capillary to investigate the atomic structure of water trapped inside. They used high magnification electron microscopy that allowed them to see individual water molecules, and the nano-capillary was made from graphene that is one atom thick and does not obscure electron microscopy imaging.
To their surprise, the scientists found small square crystals of ice at room temperature, provided the graphene capillaries were narrow enough, allowing no more than three atomic layers of water. Water molecules formed a square lattice, sitting along evenly spaced neat rows running perpendicular to each other. Such a flat square arrangement is completely uncharacteristic for water whose molecules always form little pyramidal structures inside all the previously known ices.
Using computer simulations, the researchers also attempted to find how common square ice is in nature. The results show that, if the layer of water is thin enough, it should form square ice independently of an exact chemical makeup of confining walls of a nanopore.
Therefore, it is likely that square ice is very common on the molecular scale and present at the tip of every microscopic crack or pore in any material.
Most read news
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.