Water on a nanoscale
Surfaces dominate - not the room
Advertisement
Researchers at the Max Planck Institute for polymer research have fundamentally questioned previous assumptions about how water behaves in atomically small spaces. Using spectroscopic methods and simulations, they investigated water that is confined to just a few molecular layers. Mischa Bonn's team found that the structure of the water remains remarkably "normal" - until it is limited to less than one nanometer, i.e. much thinner than previously assumed.
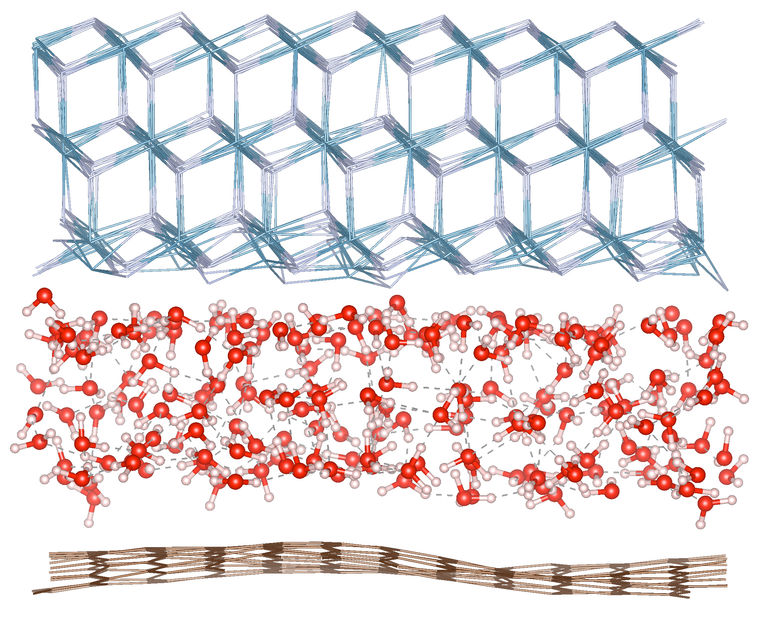
Visualization of trapped water: The figure shows a layer of water molecules (middle) trapped between the atomic lattice of calcium fluoride (CaF₂, top) and a graphene layer (bottom).
The challenge: examining only a few layers of water
Capturing the structure of a layer of water that is only a few molecules thick is an enormous scientific challenge. The team developed a nanoscale capillary device by trapping water between a single layer of graphene and a calcium fluoride (CaF₂) substrate. To reveal the microscopic structure of the trapped water, they used state-of-the-art surface-specific vibrational spectroscopy - including the orientation and hydrogen bonding of the molecules - to "visualize" the elusive few layers of water.
The scientists found that even when limited to just three layers of molecules - a space barely wider than the molecules themselves - the water in the middle still exhibits properties like ordinary, "bulk" water in contact with two surfaces. The interactions at the interfaces, determined by the graphene layer and the CaF₂ substrate, significantly influenced the arrangement and behavior of the molecules. Only at true Angstrom dimensions, i.e. at a thickness of less than two molecular layers, did the spatial boundary itself begin to structurally reorganize the water. Simulations based on machine learning confirmed the assumptions of the spectroscopic measurements, reproduced the observations and confirmed the conclusions.
"This research changes our view of confined water," explains first author Yongkang Wang. "Our results are relevant for many practical applications - such as water in nanochannels, membranes or between layers of materials - where the surfaces determine the properties of the water, not the spatial confinement itself, except for extremely thin layers on a molecular scale."
Major significance for technology, biology and materials research
These findings have major implications for numerous areas - from nanofluidics and geology to biology and materials research. The results make it clear that nano-confined water - whether on earth or in technical devices such as membranes, nanofluidic circuits or biological pores - is largely influenced by surface effects, even when strongly confined. Only for water layers less than one nanometer thick do the physical rules change fundamentally.
A new benchmark for water research
"Our results set a new benchmark," said last author Yuki Nagata. "Anyone working with so-called 'nano-confined water' should know: It's the surface chemistry - not just the geometry - that determines its properties, unless the confinement reaches the outermost limit."
The ability to specifically study and understand just a few layers of water molecules - that region of greatest scientific and technological uncertainty - represents a significant advance in water research. The research findings not only settle theoretical disputes, but also pave the way for the development of future nanodevices, materials and possibly even methods to precisely control water properties.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Wang, Y.; Tang, F.; Yu, X.; Chiang, K.; Yu, C.; Ohto, T.; Chen, Y.; Nagata, Y.; Bonn, M.; "Interfaces govern the structure of angstrom-scale confined water solutions"; Nature Communications
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!
































































