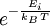To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Boltzmann factorIn physics, the Boltzmann factor is a weighting factor that determines the relative probability of a state i in a multi-state system in thermodynamic equilibrium at temperature T. Product highlightWhere kB is Boltzmann's constant, and Ei is the energy of state i. The ratio of the probabilities of two states is given by the ratio of their Boltzmann factors. The Boltzmann factor is not a probability by itself, because it is not normalized. To normalize the Boltzmann factor into a probability, one divides it by the sum Z of the Boltzmann factors of all possible states of a system, which is called the partition function. This gives the Boltzmann distribution. From the Boltzmann factor it is possible to derive the Maxwell-Boltzmann statistics, Bose-Einstein statistics, and Fermi-Dirac statistics that govern classical particles as well as quantum mechanical bosons, and fermions, respectively. See also
References
Categories: Thermodynamics | Statistical mechanics |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Boltzmann_factor". A list of authors is available in Wikipedia. |