COSMO is the abbreviation for "Conductor-like Screening Model", a calculation method for determining the electrostatic interaction of a molecule with a solvent.
In COSMO the solvent is treated as a continuum with a permittivity 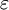 , and therefore belongs to the "continuum solvation" group of models. Also it is assumed that the medium reaches up to the "surface" of the solvated molecule. This interface is assumed to consist of spheres that surround the individual atoms (Van der Waals radius of the atoms plus a fixed distance for the solvent molecules). For the actual calculation this surface is approximated by planar pieces, e.g., triangles. , and therefore belongs to the "continuum solvation" group of models. Also it is assumed that the medium reaches up to the "surface" of the solvated molecule. This interface is assumed to consist of spheres that surround the individual atoms (Van der Waals radius of the atoms plus a fixed distance for the solvent molecules). For the actual calculation this surface is approximated by planar pieces, e.g., triangles.
If the solvent were an ideal conductor the electric potential of this surface must disappear. If the distribution of the electric charge in the molecule is known then it should be possible to calculate the charge q * on the surface. For real solvents one can assume that the charge q is lower by a factor f:
- q = fq * .
The factor f is approximately
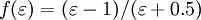
where the summand 0.5 in the denominator is an empirically determined magnitude.
From the thus determined solvent charges q and the known charge distribution of the molecule, the energy of the interaction between the solvent and the solvated molecule can be calculated.
The COSMO method can be used for all methods in theoretical chemistry where the charge distribution of a molecule can be determined, for example semiempirical calculations, Hartree-Fock-method calculations or density functional theory (quantum physics) calculations.
Comparison with other methods
While models based on the multipole expansion of the charge distribution of a molecule are limited to small, quasi-spherical or ellipsoidal molecules, the COSMO method has the advantage that it can be applied to large and irregularly formed molecular structures.
The COSMO method is more accurate for solvents with a higher permittivity because a solvent with infinite permittivity behaves like an ideal conductor. With water (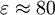 ) a very good accuracy is achieved. For solvents with low permittivities a complete solution of the electrostatic equations would be more accurate, though this would require greater effort. ) a very good accuracy is achieved. For solvents with low permittivities a complete solution of the electrostatic equations would be more accurate, though this would require greater effort.
In contrast to molecular dynamic calculations in which the motion of the molecules is calculated and the position and density averaged over time, the COSMO model, as is the case with all continuum models, has the advantage of a substantial lower computational effort.
Literature
- Fundamentals of the method:
A. Klamt, G. Schürmann, Journal of the Chemical Society, Perkin Transaction 2, 799 (1993) [1]
Source: Translated from German Wikipedia
|





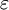 , and therefore belongs to the "continuum solvation" group of models. Also it is assumed that the medium reaches up to the "surface" of the solvated molecule. This interface is assumed to consist of spheres that surround the individual atoms (
, and therefore belongs to the "continuum solvation" group of models. Also it is assumed that the medium reaches up to the "surface" of the solvated molecule. This interface is assumed to consist of spheres that surround the individual atoms (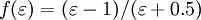
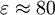 ) a very good accuracy is achieved. For solvents with low permittivities a complete solution of the electrostatic equations would be more accurate, though this would require greater effort.
) a very good accuracy is achieved. For solvents with low permittivities a complete solution of the electrostatic equations would be more accurate, though this would require greater effort.


