To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Coal gas
Coal gas is an flammable gaseous fuel made from coal and supplied to the user via a piped distribution system. Town gas is a more general term referring to manufactured gaseous fuels produced for sale to consumers and municipalities. It is also known as manufactured gas, syngas (SNG), hygas, and producer gas in some countries. Prior to the development of natural gas supplies and transmission systems during 1940s and 1950s, virtually all fuel and lighting gas used in both the United States and Great Britain was manufactured from coal. Depending on the processes used for its creation, coal gas is a mixture of the calorific gases: hydrogen, carbon monoxide, methane and volatile hydrocarbons, with small amounts of noncalorific gases - carbon dioxide and nitrogen - as impurities. Coal gas plants, especially those that operated in the past, are commonly referred to, by environmental professionals and within the utility industry, as Manufactured Gas Plants or "MGPs." Originally a by-product of the coking process, coal gas was extensively exploited in the 19th and early 20th Centuries for lighting, cooking and heating. The development of manufactured gas paralleled that of the industrial revolution and urbanization; and the byproducts, coal tars and ammonia, were at some times an important chemical feedstock for the chemical industry. Product highlightManufacturing processes
Manufactured gas can be made by two processes: carbonization or gasification. Carbonization refers to the devolatilization of an organic feedstock to yield gas and char. Gasification is the process of subjecting a feedstock to chemical reactions that produce gas.[1][2] The first process used was the carbonization and partial pyrolysis of coal. The off gases liberated in the high-temperature carbonization (coking) of coal in coke ovens were collected, scrubbed and used as fuel. Depending on the goal of the plant, the desired product was either a high quality coke for metallurgical use, with the gas being a side product or the production of a high quality gas with coke being the side product. Coke plants are typically associated with metallurgical facilities such as smelters, and blast furnaces, while gas works typically served urban areas. A facility used to manufacture coal gas, Carburetted Water Gas (CWG), and oil gas is today generally referred to as a Manufactured Gas Plant (MGP). In the early years of MGP operations, the goal of a utility gas works was to produce the greatest amount of illuminating gas. The illuminating power of a gas was related to amount of soot-forming hydrocarbons (“illuminants”) dissolved in it. These hydrocarbons gave the gas flame its characteristic bright yellow color. Gas works would typically use oily bituminous coals as feedstock. These coals would give off large amounts of volatile hydrocarbons into the coal gas, but would leave behind a crumbly, low-quality coke not suitable for metallurgical processes. Coal or Coke oven gas typically had a calorific value (CV) between 10 and 20 MJ/m³ (250-550 Btu/ft3 (std)); with values around 20 MJ/m³ (550 Btu/ft3 (std)) being typical. The advent of electric lighting forced utilities to search for other markets for manufactured gas. MGPs that once produced gas almost exclusively for lighting shifted their efforts towards supplying gas primarily for heating and cooking, and even refrigeration and cooling. Gas for industrial useFuel gas for industrial use was made using producer gas technology. Producer gas is made by blowing air through an incandescent fuel bed (commonly coke or coal) in a gas producer. The reaction of fuel with insufficient air for total combustion produces carbon monoxide (CO); this reaction is exothermic and self sustaining. It was discovered that adding steam to the input air of a gas producer would increase the CV of the fuel gas by enriching it with CO and hydrogen (H2) produced by water gas reactions. Producer gas has a very low CV of 3.7 to 5.6 MJ/m³ (100-150 Btu/ft3 (std)); because the calorific gases CO/H2 are diluted with lots of inert nitrogen (from air) and carbon dioxide (CO2) (from combustion)
The problem of nitrogen dilution was overcome by the blue water gas (BWG) process, developed in the 1850s by Sir William Siemens. The incandescent fuel bed would be alternately blasted with air followed by steam. The air reactions during the blow cycle are exothermic, heating up the bed, while the steam reactions during the make cycle, are endothermic and cool down the bed. The products from the air cycle contain non-calorific nitrogen and are exhausted out the stack while the products of the steam cycle are kept as blue water gas. This gas is composed almost entirely of CO and H2, and burns with a pale blue flame similar to natural gas. BWG has a CV of 11 MJ/m³ (300 Btu/ft3 (std)). Because blue water gas lacked illuminants it would not burn with a luminous flame in a simple fishtail gas jet as existed prior to the discovery of the Welsbach mantle in the 1890s. Various attempts were made to enrich BWG with illuminants from gas oil in the 1860s. Gas oil (an early form of gasoline) was the flammable waste product from kerosene refining, made from the lightest and most volatile fractions (tops) of crude oil. In 1875 Thaddeus S. C. Lowe invented the carburetted water gas process. This process revolutionized the manufactured gas industry and was the standard technology until the end of manufactured gas era. A CWG generating set consisted of three elements; a producer (generator), carburettor and a super heater connected in series with gas pipes and valves. During a make run, steam would be passed through the generator to make blue water gas. From the generator the hot water gas would pass into the top of the carburetor where light petroleum oils would be injected into the gas stream. The light oils would be thermocracked as they came in contact with the white hot checkerwork fire bricks inside the carburettor. The hot enriched gas would then flow into the superheater, where the gas would be further cracked by more hot fire bricks. Early history of gas production by carbonizationThe Flemish scientist Jan Baptista van Helmont (1577–1644) discovered that a 'wild spirit' escaped from heated wood and coal, and, thinking that it 'differed little from the chaos of the ancients', he named it gas in his Origins of Medicine (c. 1609). Among several others who carried out similar experiments, were Johann Becker of Munich (c 1681) and about three years later John Clayton of Wigan, England, the latter amusing his friends by lighting, what he called, "Spirit of the Coal". William Murdoch (later known as Murdock) (1754–1839) (partner of James Watt) is reputed to have heated coal in his mother's teapot to produce gas. From this beginning, he discovered new ways of making, purifying and storing gas; illuminating his house at Redruth (or his cottage at Soho) in 1792, the entrance to the Manchester Police Commissioners premises in 1797, the exterior of the factory of Boulton and Watt in Birmingham, England, and a large cotton mill in Salford, Lancashire in 1805. Professor Jan Pieter Minckeleers lit his lecture room at the University of Louvain in 1783 and Lord Dundonald lit his house at Culross, Scotland, in 1787, the gas being carried in sealed vessels from the local tar works. In France, Phillipe Lebon patented a gas fire in 1799 and demonstrated street lighting in 1801. Other demonstrations followed in France and in the United States, but, it is generally recognised that the first commercial gas works was built by the London and Westminster Gas Light and Coke Company in Great Peter Street in 1812 laying wooden pipes to illuminate Westminster Bridge with gas lights on New Year's Eve in 1813. In 1816, Rembrandt Peale and four others established the Gas Light Company of Baltimore, the first manufactured gas company in America. In 1821, natural gas was being used commercially in Fredonia, New York. The first German gas works was built in Hannover in 1825 and by 1870 there were 340 gas works in Germany making town gas from coal, wood, peat and other materials. Working conditions in the Gas Light and Coke Company's Horseferry Road Works, London, in the 1830s were described by a French visitor, Flora Tristan, in her Promenades Dans Londres: Two rows of furnaces on each side were fired up; the effect was not unlike the description of Vulcan's forge, except that the Cyclops were animated with a divine spark, whereas the dusky servants of the English furnaces were joyless, silent and benumbed. ... The foreman told me that stokers were selected from among the strongest, but that nevertheless they all became consumptive after seven or eight years of toil and died of pulmonary consumption. That explained the sadness and apathy in the faces and every movement of the hapless men.[3] The first public piped gas supply was to 13 gas lamps, each with three glass globes along the length of Pall Mall, London in 1807. The credit for this goes to the inventor and entrepreneur Fredrick Winsor and the plumber Thomas Sugg who made and laid the pipes. Digging up streets to lay pipes required legislation and this delayed the development of street lighting and gas for domestic use. Meanwhile William Murdock and his pupil Samuel Clegg were installing gas lighting in factories and work places, encountering no such impediments. Early history of gas production by gasification1850s: Gas producers invented, water gas process discovered. Mond Gas: 1850s Europeans discover that using coal instead of coke in a producer results in producer gas that contains ammonia and coal tar, Ludwig Mond's Mond Gas is processed to recover these valuable compounds. 1860s: Enrichment of BWG with illuminants from gas oil circa 1860s. Gas Oils, the volatile fractions that evaporate above kerosene, are a major problem for kerosene industry. 1875: The invention of the Carburetted Water gas process by Prof. TSC Lowe in 1875. The gas oil is fixed into the BWG via thermocracking in the carburettor and superheater of the CWG generating set. CWG is the dominant technology from 1880s until 1950s, replacing coal gasification. CWG has a CV of 20 MJ/m³ i.e slightly more than half that of natural gas. Golden age of gas light develops with the Welsbach mantle. The uses of gas and the later development of the gas industry in UKThe advent of incandescent gas lighting in factories, homes and in the streets, replacing oil lamps and candles with steady clear light, almost matching daylight in its colour, turned night into day for many—making night shift work possible in industries where light was all important—in spinning, weaving and making up garments etc. The social significance of this change is difficult for generations brought up with lighting after dark available at the touch of a switch to appreciate. Not only was industrial production accelerated, but streets were made safe, social intercourse facilitated and reading and writing made more widespread. Gas works were built in almost every town, main streets were brightly illuminated and gas was piped in the streets to the majority of urban households. The invention of the gas meter and the pre-payment meter in the late 1880s played an important role in selling town gas to domestic and commercial customers. The education and training of the large workforce, the attempts to standardise manufacturing and commercial practices and the moderating of commercial rivalry between supply companies prompted the founding of associations of gas managers, first in Scotland in 1861. A British Association of Gas Managers was formed in 1863 in Manchester and this, after a turbulent history, became the foundation of the Institute of Gas Engineers (IGE). In 1903, the reconstructed Institution of Civil Engineers (IGE) initiated courses for students of gas manufacture in the City and Guilds of London Institute. The IGE was granted the Royal Charter in 1929. Universities were slow to respond to the needs of the industry and it was not until 1908 that the first Professorship of Coal Gas and Fuel Industries was founded at the University of Leeds. In 1926, the Gas Light and Coke Company opened Watson House adjacent to Nine Elms Gas Works. At first, this was a scientific laboratory. Later it included a centre for training apprentices but its major contribution to the industry was its gas appliance testing facilities, which were made available to the whole industry, including gas appliance manufacturers. Using this facility, the industry established not only safety but also performance standards for both the manufacture of gas appliances and their servicing in customers' homes and commercial premises. During World War I, the gas industry's by-products, phenol, toluene and ammonia and sulphurous compounds were valuable ingredients for explosives. Much coal for the gas works was shipped by sea and was vulnerable to enemy attack. The gas industry was a large employer of clerks, mainly male before the war. But the advent of the typewriter and the female typist made another important social change that was, unlike the employment of women in war-time industry, to have long lasting effects. The inter-war years were marked by the development of the continuous vertical retort which replaced many of the batch fed horizontal retorts. There were improvements in storage, especially the waterless gas holder, and distribution with the advent of 2 - 4 inch steel pipes to convey gas at up to 50 psi as feeder mains to the traditional cast iron pipes working at an average of 2 - 3 inches water gauge. Benzole as a vehicle fuel and coal tar as the main feedstock for the emerging organic chemical industry provided the gas industry with substantial revenues. Petroleum supplanted coal tar as the primary feedstock of the organic chemical industry after World War II and the loss of this market contributed to the economic problems of the gas industry after the war. A wide variety of appliances and uses for gas developed over the years. Gas fires, gas cookers, refrigerators, washing machines, hand irons, pokers for fire lighting, gas-heated baths, remotely controlled clusters of gas lights, gas engines of various types and, in later years, gas warm air and hot water central heating and air conditioning, all of which made immense contributions to the improvement of the quality of life in cities and towns world wide. The evolution of electric lighting made available from public supply extinguished the gas light, except where colour matching was practised as in haberdashery shops. The post-war house building programme put gas at a disadvantage. Whereas electricity had long developed a national distribution grid, which enabled supplies to reach even small new housing developments, gas was still distributed only locally. Many new housing estates were beyond the reach of the gas main and the stringent Treasury rules about return on investment made extension of mains uneconomic. Electricity made inroads into the home heating market with underfloor heating and night storage heaters using cheap off-peak electricity supplies. By the 1960s, manufactured gas, compared with its main rival in the energy market, electricity, was considered "nasty, smelly, dirty and dangerous (to quote market research of the time) and seemed doomed to lose market share still further, except for cooking where its controllability gave it marked advantages over both electricity and solid fuel. The development of more efficient gas fires assisted gas to resist competition in the market for room heating. Concurrently a new market for whole house central heating by hot water was being developed by the oil industry and the gas industry followed suit. Gas warm air heating found a market niche in new local authority housing where low installation costs gave it an advantage. These developments, the realignment of managerial thinking away from commercial management (selling what the industry produced) to marketing management (meeting the needs, wants and desires of customers) and the lifting of an early moratorium preventing nationalised industries from using television advertising, saved the gas industry for long enough to provide a viable market for what was to come. Change over to natural gasIn 1959 the British Gas Council demonstrated that liquid natural gas (LNG) could be transported safely, efficiently and economically over long distances by sea. The 'Methane Pioneer' shipped a consignment of LNG from USA to a new LNG terminal on Canvey Island and customers there were converted to use the new fuel. A 320 mile long high pressure trunk pipeline was built from London to Leeds. The slow death of the town gas industry in the UK was signalled by the discovery of natural gas, by the ill-fated BP drilling rig Sea Gem on 17 September 1965 some forty miles off Grimsby, over 8000 feet below the sea bed. Subsequently the North Sea was found to have many rich gas fields on both sides of the median line which defined which nations should have rights over the reserves. The Fuel Policy White paper of 1967 (Cmd. 3438) pointed the industry in the direction of building up the use of natural gas speedily to 'enable the country to benefit as soon as possible from the advantages of this new indigenous energy source'. As a result there was a 'rush to gas' for use in peak load electricity generation and in low grade uses in industry. The effects on the coal industry were very significant; not only did coal lose its market for town gas production, it came to be displaced from much of the bulk energy market also. The exploitation of the North Sea gas reserves, entailing landing gas at Easington, Bacton and St Fergus made viable the building of a national distribution grid, of over 3000 miles, consisting of two parallel and interconnected pipelines running the length of the country. All gas equipment in the whole of UK was converted (by the fitting of different-sized burner jets to give the correct gas/air mixture) from burning town gas to burn natural gas (mainly methane) over the period from 1967 to 1977 at a cost of about £100 million including the writing off of redundant town gas manufacturing plants. All the gas using equipment of almost 13 million domestic, 400 thousand commercial and 60 thousand industrial customers was converted. Many dangerous appliances were discovered in this exercise and were taken out of service. The British town gas industry died on 1st September 1977 when the last town gas burning appliances were converted to natural gas in Edinburgh. As well as requiring little processing before use, natural gas is non-toxic; the carbon monoxide (CO) in town gas made it extremely poisonous, accidental poisoning and suicide by gas being commonplace. Poisoning from natural gas appliances is only due to incomplete combustion, which creates CO, and flue leaks to living accommodation. As with town gas, a small amount of foul-smelling substance (mercaptan) is added to the gas to indicate to the user that there is a leak or an unlit burner, the gas having no odour of its own. The organisation of the British gas industry adapted to these changes, first, by the Gas Act 1965 by empowering the Gas Council to acquire and supply gas to the twelve area Boards. Then, the Gas Act 1972 formed the British Gas Corporation as a single commercial entity, embracing all the twelve Area Gas Boards, allowing them to acquire, distribute and market gas and gas appliances to industrial commercial and domestic customers throughout the UK. In 1986, British Gas was privatised and dismembered and the Government no longer has any direct control over it. The most recent demergers are described at http://www.britishgas.co.uk/ During the era of North Sea gas, much of the original cast irongas pipes installed in towns and cities for town gas have been replaced by plastic. As reported in the DTI Energy Review 'Our Energy Challenge' January 2006 North Sea gas resources have been depleted at a faster rate than had been anticipated and gas supplies for the UK are being sought from remote sources: a strategy made possible by developments in the technologies of pipelaying that enable the transmission of gas over land and under sea across and between continents. Natural gas is now a world commodity. Such sources of supply are exposed to all the risks of any import. There are still substantial coal reserves in UK and this fact prompts the thought that at some time in the future, coal gas may once again be a reliable indigenous source of energy. Development of Pacific coast oil gas processMassive problems with lampblack created from the Pacific coast process. Up to 20 to 30 lb/1000 ft³ (300 to 500 g/m³) of oily soot. Major pollution problem leads to passage of early environmental legislation at the state level. Layout of a typical gas plant
Issues in gas processing
WWI-interwar era developments
Post WWII: the decline of manufactured gas
Post WWII positive developments
Environmental effectsFrom its original development until the wide scale adoption of natural gas, more than 50,000 manufactured gas plants were in existence in the United States alone. The process of manufacturing gas usually produced a number of by-products that contaminated the soil and groundwater in and around the manufacturing plant, so many former town gas plants are a serious environmental concern, and cleanup and remediation costs are often high. MGPs were typically sited near or adjacent to waterways that were used for the discharge of wastewater contaminated with tar, ammonia and/or drip oils, as well as outright waste tars and tar-water emulsions. In the earliest days of MGP operations, coal tar was considered a waste and often disposed into the environment in and around the plant locations. While uses for coal tar developed by the late-1800s, the market for tar varied and plants that could not sell tar at a given time could either store tar for future use, attempt to burn it as fuel for the boilers, or dump the tar as waste. Commonly, waste tars were disposed of in old gas holders, adits or even mine shafts (if present). Over time, the waste tars degrade with phenols, benzene (and other mono-aromatics - BTEX) and polycyclic aromatic hydrocarbons released as pollutant plumes that can escape into the surrounding environment. Other wastes included "blue billy". [4] The shift to the CWG process initially resulted in a reduced output of water gas tar as compared to the volume of coal tars. The advent of automobiles reduced the availability of naphtha for carburetion oil, as that fraction was desirable as motor fuel. MGPs that shifted to heavier grades of oil often experienced problems with the production of tar-water emulsions, which were difficult, time consuming, and costly to break. [The cause of tar-water emulsions is complex and was related to several factors, including free carbon in the carburetion oil and the substitution of bituminous coal as a feedstock instead of coke.] The production of large volumes of tar-water emulsions quickly filled up available storage capacity at MGPs and plant management often dumped the emulsions in pits, from which they may or may not have been later reclaimed. Even if the emulsions were reclaimed, the environmental damage from placing tars in unlined pits remained. The dumping of emulsions (and other tarry residues such as tar sludges, tank bottoms, and off-spec tars) into the soil and waters around MGPs is a significant factor in the pollution found at FMGPs today. Commonly associated with former manufactured gas plants (known as "FMGPs" in environmental remediation) are contaminants including:
In the UK, former gasworks have commonly been developed over for residential and other uses (including the Millennium Dome), being seen as prime developable land in the confines of city boundaries. Situations such as these are now lead to problems associated with planning and the Contaminated Land Regime and have recently been debated in the House of Commons. It should be noted that the more modern coal gasification processes (circa 1970 to 2006) also have environmental problems requiring various available technologies for mitigation.[1][2] Usage in the UKIn the UK, coal gas specifically means gas made by the destructive distillation of coal. The term is not applied to other coal-derived gases, such as water gas, producer gas and syngas. US usage may be different. Coal gas was introduced in the UK in the 1790s as an illuminating gas by the Scottish inventor William Murdoch and became very widely used for lighting, cooking, heating and powering gas engines. ManufactureCoal was heated in a retort and the crude gas was passed through a condenser to remove tar and a scrubber to remove other impurities. The residue remaining in the retort was coke. CompositionThe composition of coal gas varied according to the type of coal and the temperature of carbonisation. Typical figures were:
In a plain burner, only the ethylene produced a luminous flame but the light output could be greatly increased by using a gas mantle. By-productsThe by-products of coal gas manufacture included coke, coal tar, sulfur and ammonia and these were all useful products. CokeCoke is used as a smokeless fuel and for the manufacture of water gas and producer gas Coal tarCoal tar was subjected to fractional distillation to recover various products, including
SulfurUsed in the manufacture of sulfuric acid AmmoniaUsed in the manufacture of fertilisers Structure of the industryCoal gas was initially manufactured by independent companies but many of these later became municipal services. Both the private and the municipal companies were nationalised under the The Gas Act 1948 and further re-structuring took place under The Gas Act 1972. For further details see British Gas plc. Coal gas is no longer made in the UK. It was replaced first by gas made from oil and later by natural gas from the North Sea. See also
References
Further Reading
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Coal_gas". A list of authors is available in Wikipedia. |





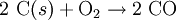 (Exothermic: Producer gas Reaction)
(Exothermic: Producer gas Reaction)
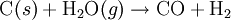 (Endothermic:
(Endothermic: 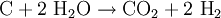 (Endothermic)
(Endothermic)
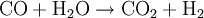 (Exothermic: Water gas shift reaction)
(Exothermic: Water gas shift reaction)


