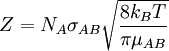To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Collision frequencyCollision frequency is defined in chemical kinetics, in the background of theoretical kinetics, as the average number of collisions between reacting molecules per unit of time. Its symbol is Z. Product highlightUnlike the preexponential factor, A, which is an empirical magnitude, Z is a theoretical magnitude, calculated ab initio. The closer A and Z are, the better a given theory of chemical kinetics is. For a gas phase bimolecular reaction Z is
References
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Collision_frequency". A list of authors is available in Wikipedia. |