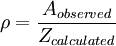To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Collision theoryCollision theory, proposed by Max Trautz and William Lewis in 1916, qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions.[1] It assumes that for a reaction to occur the reactant particles must collide, but only a certain fraction of the total collisions, the effective collisions, cause the transformation of reactant molecules into products. This is due to the fact that only a fraction of the molecules have sufficient energy and the right orientation at the moment of impact to break the existing bonds and form new bonds. The minimal amount of energy needed so that the molecule is transformed is called activation energy. Collision theory is closely related to chemical kinetics.
Product highlight
Rate constantThe rate constant for a bimolecular gas phase reaction, as predicted by collision theory is:
And the collision frequency is:
Qualitative overviewFundamentally collision theory is based on kinetic theory and therefore it can only be applied strictly to ideal gases, otherwise approximations are used. Qualitatively, it assumes that the molecules of the reactants are rigid, uncharged spheres that physically collide prior to reacting. Moreover, it postulates that the majority of collisions do not lead to a reaction, but only those in which the colliding species have:
These collisions which lead to reaction are called effective collisions. The reaction rate, may be defined as the number of effective collisions per unit time. According to collision theory, two significant factors determine reaction rates:
If a heterogeneous reaction takes place, then the surface area of the solid is also important: the more reactive centers exposed on the surface (due to the porosity of the solid and how finely divided it is), the more collisions with reacting molecules. Quantitative insightsDerivationCollision theory can only be applied quantitatively to bimolecular reactions, of the kind:[4]
In collision theory it is considered that two particles A and B will collide if their nuclei get closer than a certain distance. The area around a molecule A in which it can collide with an approaching B molecule is called the cross section (σAB) of the reaction and is, in principle, the area corresponding to a circle whose radius (rAB) is the sum of the radii of both reacting molecules, which are supposed to be spherical.
A moving molecule will therefore sweep a volume From kinetic theory it is known that a molecule of A has an average velocity (different from root mean square velocity) of The solution of the two body problem states that two different moving bodies can be treated as one body which has the reduced mass of both and moves with the velocity of the center of mass, so, in this system μAB must be used instead of mA. Therefore, the total collision frequency,[2] of all A molecules, with all B molecules, is:
From Maxwell Boltzmann distribution it can be deduced that the fraction of collisions with more energy than the activation energy is
Where:
The product Zρ is equivalent to the preexponential factor of the Arrhenius equation. Validity of the theory and steric factorOnce a theory is formulated, its validity must be tested, that is, compare its predictions with the results of the experiments. When the expression form of the rate constant is compared with the rate equation for an elementary bimolecular reaction, That expression is similar to the Arrhenius equation, and gives the first theoretical explanation for the Arrhenius equation on a molecular basis. The weak temperature dependence of the preexponential factor is so small compared to the exponential factor that it cannot be measured experimentally, that is, "it is not feasible to establish, on the basis of temperature studies of the rate constant, whether the predicted T½ dependence of the preexponential factor is observed experimentally"[4] Steric factorIf the values of the predicted rate constants are compared with the values of known rate constants it is noticed that collision theory fails to estimate the constants correctly and the more complex the molecules are, the more it fails. The reason for this is that particles have been supposed to be spherical and able to react in all directions; that is not true, as the orientation of the collisions is not always the right one. For example in the hydrogenation reaction of ethylene the H2 molecule must approach the bonding zone between the atoms, and only a few of all the possible collisions fulfill this requirement.
Usually, the more complex the reactant molecules, the lower the steric factor. Nevertheless, some reactions exhibit steric factors greater than unity: the harpoon reactions, which involve atoms that exchange electrons, producing ions. The deviation from unity can have different causes: the molecules are not spherical, so different geometries are possible; not all the kinetic energy is delivered into the right spot; the presence of a solvent (when applied to solutions), etc.
Collision theory can be applied to reactions in solution; in that case, the solvent cage has an effect on the reactant molecules and several collisions can take place in a single encounter, which leads to predicted preexponential factors being too large. ρ values greater than unity can be attributed to favorable entropic contributions.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Collision_theory". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





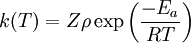 .
.
 is the steric factor.
is the steric factor.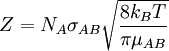
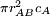 per second as it moves, where
per second as it moves, where 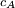 is the average velocity of the particle.
is the average velocity of the particle.
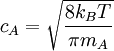 . Where
. Where 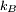 is
is 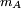 is the mass of the molecule.
is the mass of the molecule.
![N_A^{2} \sigma_{AB} \sqrt \frac{8 k_B T}{\pi \mu_{AB}}[A][B] =N_A^{2} r^{2}_{AB} \sqrt \frac{8 \pi k_B T}{ \mu_{AB}}[A][B] = Z [A][B]](images/math/d/5/7/d57521a066ff17dcd36f6b20daca62ad.png)
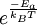 . Therefore the rate of a bimolecular reaction for ideal gases will be:
. Therefore the rate of a bimolecular reaction for ideal gases will be:
![r = Z \rho [A][B] \exp \left( \frac{-E_{a}}{RT} \right)](images/math/b/b/4/bb4fd45453eb7893a06f20e52fcf1e9e.png)
![\scriptstyle r =k(T) [A][B]](images/math/8/3/d/83d87be14e69236bd3c960b13efe888b.png) , it is noticed that
, it is noticed that 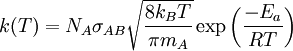 .
.