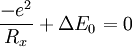To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Harpoon reactionHarpoon reactions[1] are a type of chemical reaction between two substances one of them prone to form a cation, generally a metal, and the other one prone to form an anion, generally a halogen. Product highlightTheir main feature is that these reactions, unlike most reactions, have steric factors greater than unity, that is, they take place faster than predicted by collision theory. This is explained by the fact that the colliding particles have greater cross sections than the pure geometrical ones calculated from their radii, because when the particles are close enough, an electron "jumps" (therefore the name) from one of the particles to the other one, forming an anion and a cation which subsequently attract each other. Harpoon reactions usually take place in the gas phase, but they are also possible in condensed media.[2][3] The calculated rate constant can be improved by using a better estimation of the steric factor. A rough approximation is that the largest separation Rx at which charge transfer can take place on energetic grounds, can be estimated from the solution of the following equation that determines the largest distance at which the Coulombic attraction between the two oppositely charged ions is sufficient to provide the energy ΔE0
Examples of harpoon reactions
References
Categories: Chemical kinetics | Chemical reactions |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Harpoon_reaction". A list of authors is available in Wikipedia. |