To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Free neutron
A free neutron is a neutron that exists outside of an atomic nucleus. While neutrons can be stable when bound inside nuclei, free neutrons are unstable and decay with a lifetime of just under 15 minutes (885.7 ± 0.8 s).[1] The only possible decay mode, via the weak nuclear force, is into a proton, an electron, and an electron antineutrino (antineutrino), the proton and electron forming a hydrogen atom: 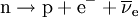 Even though it is not a chemical element, the free neutron is often included in tables of nuclides. It is then considered to have an atomic number of zero and a mass number of one. Product highlight
ProductionVarious nuclides become more stable by expelling neutrons as a decay mode; this is known as neutron emission, and happens commonly during spontaneous fission. Cosmic radiation interacting the earth's atmosphere continuously generates neutrons that can be detected at the surface. Nuclear fission reactors naturally produce free neutrons; their role is to sustain the energy-producing chain reaction. The intense neutron radiation can also be used to produce various radioisotopes through the process of neutron activation, which is a type of neutron capture. Experimental nuclear fusion reactors produce free neutrons as a waste product. However, it is these neutrons that possess most of the energy, and converting that energy to a useful form has proved a difficult engineering challenge to nuclear physicists. This also explains why this form of energy is likely to create around twice the amount of radioactive waste of a fission reactor, but with a short (50-100 years) decay period (as opposed to the 10,000 years for fission waste). [1] [2] Thermal neutronA thermal neutron is a free neutron that is Boltzmann distributed with kT = 0.024 eV (4.0×10-21 J) at room temperature. This gives characteristic (not average, or median) speed of 2.2 km/s. The name 'thermal' comes from their energy being that of the room temperature gas or material they are permeating. (see kinetic theory for energies and speeds of molecules). After a number of collisions (often in the range of 10–20) with nuclei, neutrons arrive at this energy level, provided that they are not absorbed. In many substances, thermal neutrons have a much larger effective cross-section than faster neutrons, and can therefore be absorbed more easily by any atomic nuclei that they collide with, creating a heavier — and often unstable — isotope of the chemical element as a result. Most fission reactors use a neutron moderator to slow down, or thermalize the neutrons that are emitted by nuclear fission so that they are more easily captured, causing further fission. Others, called fast breeder reactors, use fission energy neutrons directly. Cold neutronsThese neutrons are thermal neutrons that have been equilibrated in a very cold substances such as liquid deuterium. These are produced in neutron scattering research facilities. Fission energy neutronA fast neutron is a free neutron with a kinetic energy level close to 2 MeV (20 TJ/kg), hence a speed of 28,000 km/s. They are named fission energy or fast neutrons to distinguish them from lower-energy thermal neutrons, and high-energy neutrons produced in cosmic showers or accelerators. Fast neutrons are produced by nuclear processes such as nuclear fission. Fast neutrons can be made into thermal neutrons via a process called moderation. This is done with a neutron moderator. In reactors, typically heavy water, light water, or graphite are used to moderate neutrons. Fusion neutrons can have higher energies such as 14.1 MeV for D-T fusion, or 2.45 MeV for D-D fusion to 3He. See Nuclear fusion#Criteria and candidates for terrestrial reactions for a list. Intermediate neutronsA fission energy neutron that is slowing down is often said to have intermediate energy. There are not many non-elastic reactions in this energy region, so most of what happens is just slowing to thermal speeds before eventual capture. Intermediate energy neutrons are a hazard in reactors owing to the existence of a resonance region in the fission cross section of fissile elements. Within this region there exist many local minima and local maxima of probability of causing fission; this means that a reactor operating with a significant population of intermediate neutrons in contact with fuel nuclei could exhibit dangerous transient response. In such reactors, other mechanisms of inherent stability must be provided, such as large hydrogen populations to provide Doppler broadening. High-energy neutronsThese neutrons have more energy than fission energy neutrons and generated in accelerators or in the atmosphere from cosmic particles. They can have energies as high as tens of joules per neutron. See also
References
|
|||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Free_neutron". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||







