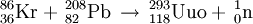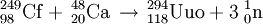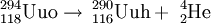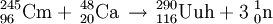To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Ununoctium
Ununoctium (pronounced /ˌjuːnəˈnɒktiəm/ [6][7]) is the temporary IUPAC name for the transactinide element having atomic number of 118. It can also be referred to as eka-radon and it is currently the only synthetic member of the noble gases group, and the highest atomic number assigned to a reputedly discovered element. Its temporary IUPAC element symbol is Uuo. Only three atoms of this element have been detected.[8] The name ununoctium is a systematic element name, used as a placeholder until it will be confirmed by other research groups and the IUPAC decides on a name. Moskowium (Mk) has been proposed by their Russian discoverers as the permanent name for the element.[9][10] Product highlight
IsolationUnsuccessful attemptsIn late 1998, the Polish theoreticist Robert Smolanczuk published some calculations on the fusion of atomic nuclei towards the synthesis of superheavy atoms, including the element 118.[11] His calculations suggested that it might be possible to make element 118 by fusing lead with krypton under carefully controlled conditions.[12] In 1999, researchers at Lawrence Berkeley National Laboratory made use of these predictions and announced the discovery of elements 116 and 118, in a paper published in Physical Review Letters,[13] and very soon after the results were reported in Science.[14] The researchers claimed to have performed the reaction: The following year, they published a retraction after other researchers were unable to duplicate the results.[15] In June 2002, the director of the lab announced that the original claim of the discovery of these two elements had been based on data fabricated by principal author Victor Ninov.[16] The American group had intended to name it ghiorsium after Albert Ghiorso (a member of the research team) before having to retract their claim.[17] DiscoveryOn October 16, 2006, researchers from Joint Institute for Nuclear Research (JINR) and Lawrence Livermore National Laboratory of California, USA, working at the JINR in Dubna, Russia, announced in Physical Review C that they had indirectly detected a total of three nuclei of ununoctium-294 (one in 2002[18] and two more in 2005) produced via collisions of californium-249 atoms and calcium-48 ions:[19][20][21][22][23]
Because of the very small fusion reaction probability (the fusion cross section is 0.5 pb = 5×10−41 m2) the experiment took 4 months and involved a beam of 4×1019 calcium ions that had to be shot at the californium target to produce the single recorded event believed to be the synthesis of the element 118.[1] Nevertheless, researchers are highly confident that the result is not a false positive, since the chance that the detection was a random event was estimated to be less than one part in 100,000.[24] In the experiment, the decay products of three atoms of ununoctium, rather than the atoms themselves, were observed. A half-life of 0.89 ms was observed: 294Uuo decays into 290Uuh by alpha decay. Since there were only three nuclei, the half-life was derived from observed lifetimes and has a large uncertainty: 0.89-0.31+1.07 ms.[5] The identification of the 294Uuo nuclei was verified by separately creating the putative daughter nucleus 290Uuh by means of a bombardment of 245Cm with 48Ca ions, and checking that the 290Uuh decay matched the decay chain of the 294Uuo nuclei.[5] The daughter nucleus 290Uuh is very unstable, decaying with a half-life of 14 milliseconds into 286Uuq, which may undergo spontaneous fission or alpha decay into 282Uub, which will undergo spontaneous fission.[5] Characteristics
StabilityAlthough the half-life seems to be less than a millisecond, it is still longer than predicted, thus giving further support to the idea of “island of stability.”[25] This concept, proposed by late UC Berkeley professor Glenn Seaborg, explains why superheavy elements such as element 118 last longer than predicted.[26] ChemistryUnunoctium has all its electrons in a closed shell which makes it a member of the noble gas group. Therefore it is most likely that it will have similar properties as other members of the group, resembling in its chemical properties the noble gas above it in the periodic table, radon.[27] There are no known compounds of ununoctium, and because it is likely to have a high ionization energy, the most common oxidation state will likely be 0.[4] It is believed to be a gas under normal conditions,[2] thus making it one of the gaseous substances with the highest molecular masses — in fact, only uranium hexafluoride (UF6) with a molecular mass of 352 would surpass it. Since only three atoms of ununoctium have ever been produced, it currently has no uses outside of basic scientific research. Also, because it does not occur at all in the biosphere it never presents a risk, but it would constitute a radiation hazard if enough was ever assembled in one place.[28]
References
See alsoCategories: Chemical elements | Noble gases |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Ununoctium". A list of authors is available in Wikipedia. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||