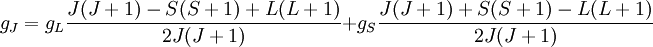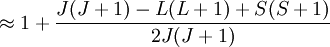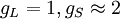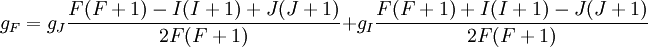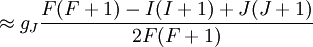To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Landé g-factorIn physics, the Landé g-factor is a particular example of a g-factor, namely for an electron with both spin and orbital angular momenta. It is named after Alfred Landé, who first described it in 1921. Product highlightIn atomic physics, it is a multiplicative term appearing in the expression for the energy levels of an atom in a weak magnetic field. The quantum states of electrons in atomic orbitals are normally degenerate in energy, with the degenerate states all sharing the same angular momentum. When the atom is placed in a weak magnetic field, however, the degeneracy is lifted. The factor comes about during the calculation of the first-order perturbation in the energy of an atom when a weak uniform magnetic field (that is, weak in comparison to the system's internal magnetic field) is applied to the system. Formally we can write the factor as, Here, J is the total electronic angular momentum, L is the orbital angular momentum, and S is the spin angular momentum. Because S=1/2 for electrons, one often sees this formula written with 3/4 in place of S(S+1). The quantities gL and gS are other g-factors of an electron, as defined in the article on g-factors. If we wish to know the g-factor for an atom with total atomic angular momentum F=I+J, This last approximation is justified because gI is smaller than gJ by the ratio of the electron mass to the proton mass. |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Landé_g-factor". A list of authors is available in Wikipedia. |