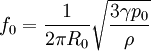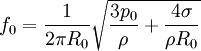To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Liquid bubble
Due to surface tension, bubbles may remain intact when they reach the surface of the immersive substance. Product highlight
Common examples
Bubbles are seen in many places in everyday life, for example:
Physics and chemistry of bubblesBubbles form, and coalesce into globular shapes, because those shapes are at a lower energy state. For the physics and chemistry behind it, see nucleation. The appearance of bubblesHumans can see bubbles because they have a different refractive index (IR) than the surrounding substance. For example, the IR of air is approximately 1.0003 and the IR of water is approximately 1.333. Snell's Law describes how electromagnetic waves change direction at the interface between two mediums with different IR; thus bubbles can be identified from the accompanying refraction and internal reflection even though both the immersed and immersing mediums are transparent. One should note that the above explanation only holds for bubbles of one medium submerged in another medium (e.g. bubbles of air in a soft drink); the volume of a membrane bubble (e.g. soap bubble) will not distort light very much, and one can only see a membrane bubble due to thin-film diffraction and reflection. ApplicationsNucleation can be intentionally induced, for example to create bubblegram art. PulsationWhen bubbles are disturbed, they pulsate (that is, they oscillate in size) at their natural frequency. Large bubbles (negligible surface tension and thermal conductivity) undergo adiabatic pulsations, which means that no heat is transferred either from the liquid to the gas or vice versa. The natural frequency of such bubbles is determined by the equation:[1][2] where:
Smaller bubbles undergo isothermal pulsations. The corresponding equation for small bubbles of surface tension σ (and negligible liquid viscosity) is[2] Excited bubbles trapped underwater are the major source of liquid sounds, such as when a rain droplet impacts a surface of water.[3][4] References
See also
Categories: Fluid mechanics | Bubbles |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Liquid_bubble". A list of authors is available in Wikipedia. |