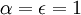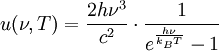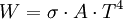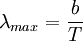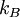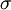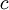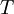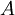To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Thermal radiation
Thermal radiation is electromagnetic radiation emitted from the surface of an object which is due to the object's temperature. Infrared radiation from a common household radiator or electric heater is an example of thermal radiation, as is the light emitted by a glowing incandescent light bulb. Thermal radiation is generated when heat from the movement of charged particles within atoms is converted to electromagnetic radiation. The emitted wave frequency of the thermal radiation is a probability distribution depending only on temperature, and for a genuine black body is given by Planck’s law of radiation. Wien's law gives the most likely frequency of the emitted radiation, and the Stefan-Boltzmann law gives the heat intensity. Product highlight
PropertiesThere are three main properties that characterize thermal radiation:
Interchange of energyThermal radiation is an important concept in thermodynamics as it is partially responsible for heat exchange between objects, as warmer bodies radiate more heat than colder ones. (Other factors are convection and conduction.) The interplay of energy exchange is characterized by the following equation: Here, In a practical situation and room-temperature setting, objects lose considerable energy due to thermal radiation. However, the energy lost by emitting infrared heat is regained by absorbing the heat of surrounding objects. For example, a human being, roughly 2 square meter in area, and about 307 kelvins in temperature, continuously radiates about 1000 watts. However, if people are indoors, in a room of 296 K, they receive back about 900 watts from the wall, ceiling, and other surroundings, so the net loss is only about 100 watts. Clothes (having poorer thermal conductivity than human skin, therefore reducing the speed of heat loss from the human body to surrounding environment) reduce this loss still further. If objects appear white (reflective in the visual spectrum), they are not necessarily equally reflective (and thus non-emissive) in the thermal infrared; e. g. most household radiators are painted white despite the fact that they have to be good thermal radiators. Acrylic and urethane based white paints have 93% blackbody radiation efficiency at room temperature (meaning the term "black body" does not always correspond to the visually perceived color of an object). Calculation of radiative heat transfer between groups of object, including a 'cavity' or 'surroundings' requires solution of a set of simultaneous equations using the Radiosity method. In these calculations, the geometrical configuration of the problem is distilled to a set of numbers called view factors, which give the proportion of radiation leaving any given surface that hits another specific surface. These calculations are important in the fields of Solar thermal energy, Boiler and Furnace design and raytraced computer graphics. FormulaThermal radiation power of a black body per unit of area, unit of solid angle and unit of frequency ν is given by This formula mathematically follows from calculation of spectral distribution of energy in quantized electromagnetic field which is in complete thermal equilibrium with the radiating object. Integrating the above equation over ν the power output given by the Stefan-Boltzmann law is obtained, as: Further, the wavelength For surfaces which are not black bodies, one has to consider the (generally frequency dependent) emissivity correction factor ε(υ). This correction factor has to be multiplied with the radiation spectrum formula before integration. The resulting formula for the power output can be written in a way that contains a temperature dependent correction factor which is (somewhat confusingly) often called ε as well: ConstantsDefinitions of constants used in the above equations:
See also
Categories: Electromagnetic radiation | Thermodynamics |
||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Thermal_radiation". A list of authors is available in Wikipedia. |





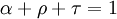
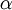 represents spectral absorption factor,
represents spectral absorption factor, 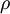 spectral reflection factor and
spectral reflection factor and 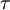 spectral transmission factor. All these elements depend also on the wavelength
spectral transmission factor. All these elements depend also on the wavelength 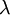 . The spectral absorption factor is equal to the
. The spectral absorption factor is equal to the 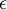 ; this relation is known as
; this relation is known as