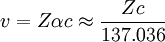To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Untriseptium
Untriseptium (pronounced /ˌʌntraɪˈsɛptiəm/) is a chemical element which has not yet been observed to occur naturally or be synthesised. Its atomic number is 137 and symbol is Uts. Product highlightThe name untriseptium is a temporary IUPAC systematic element name. HistoryThe name untriseptium is used as a placeholder, as in scientific articles about the search for element 137. Transuranic elements (those beyond uranium) are, except for microscopic quantities and except for plutonium, always artificially produced, and usually end up being named for a scientist or the location of a laboratory that does work in atomic physics (see systematic element name for more information).
SignificanceIn a non-relativistic approximation, the speed of an electron in a 1s electron orbital, v, can be obtained using the expression: where Z is the atomic number, and α is the fine structure constant, a measure of the strength of electromagnetic interactions. Under this approximation, any element with an atomic number of greater than 137 would require 1s electrons to be traveling faster than c, the speed of light. A complete analysis involving relativity reduces the speed of electrons, therefore allowing stable 1s orbits in the element 138 (Uto). See also
|
|||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Untriseptium". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||