How Spheres Become Worms: Breakthrough with NMR spectroscopy
A previously unknown form of hydrogel formation has been elucidated: chemists found unusual interactions between polymers
Advertisement
Hydrogels? Many people use these substances without knowing it. As superabsorbents in nappies, for example, hydrogels absorb a lot of liquid. In the process, the initially dry material becomes Jelly-like, but it does not wet. Some people place the swellable material on their eyeballs – soft contact lenses are also just hydrogels. The same goes for jelly and other everyday materials.
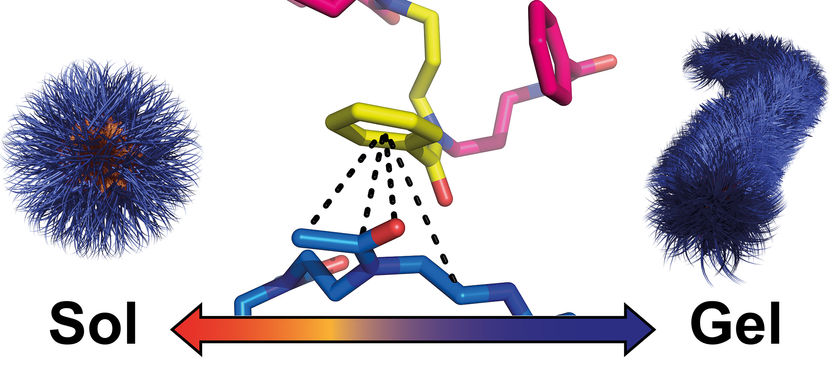
Specific interactions cause spherical nanoparticles of polymers to rearrange themselves into worm-like structures during cooling. The resulting hydrogel dissolves again when heated.
Theresa Zorn, University of Würzburg
Hydrogels also play a role in science. From a chemical point of view, they are long, three-dimensionally cross-linked polymer molecules that form cavities. Inside, they can absorb and hold water molecules.
In the working group of former Würzburg chemistry professor Robert Luxenhofer, the suitability of hydrogels for biofabrication is being tested: For example, hydrogels can be used for 3D printing as scaffold structures, on which cells can be attached. In this way, for example, artificial tissues can be produced for medical research and regenerative therapies.
Hydrogel formation posed a puzzle
During this research, Dr. Lukas Hahn in Luxenhofer's team noticed an unusual form of hydrogel formation. He observed it in polymers intended for nanomedicine, specifically for drug delivery.
These polymers arrange themselves into spherical nanoparticles in water at 40 degrees. When the water is cooled to below 32 degrees, the spheres cluster into worm-like structures and a gel is formed. When heated, it dissolves again.
"This behaviour is very rare in synthetic polymers and was completely unexpected," explains Robert Luxenhofer, who now teaches and researches at the University of Helsinki. If it does occur, the gel formation is usually due to hydrogen bonds – attractive forces between polar functional groups involving hydrogen atoms that have a stabilising effect. Such interactions are of central importance for the structure and function of proteins, for example.
However, things are quite different with the polymers we are dealing with here. In terms of their chemical structure, they are not capable of forming hydrogen bonds with each other. Apparently, the researchers had stumbled upon an unknown mechanism of gel formation.
Breakthrough with NMR spectroscopy
To solve the puzzle, Robert Luxenhofer sought a cooperation with chemistry professor Ann-Christin Pöppler at Julius-Maximilians-Universität Würzburg (JMU), an expert in the characterisation of nanoparticles made of polymers. In cooperation with other research groups, her team took a closer look at the peculiar form of gel formation – a complex puzzle that took a good two years to solve.
"We were able to elucidate the unknown mechanism because we used a wide variety of analytical tools. In the end, however, the breakthrough came with various methods of NMR spectroscopy," explains the JMU chemist. Her doctoral student Theresa Zorn found out what leads to gel formation in this case: specific interactions between amide groups of the water-soluble and phenyl rings of the non-water-soluble polymer building blocks. These interactions cause the spherical nanoparticles to condense and restructure into worm-like structures.
The findings could be confirmed by theoretical calculations: Dr Josef Kehrein, a former PhD student of JMU professor Christoph Sotriffer, an expert in computer-aided modelling of three-dimensional interactions between molecules, succeeded in doing so. He, too, is now working in Helsinki.
The results have been published in ACS Nano, a journal of the American Chemical Society (ACS). The German Research Foundation (DFG), the Academy of Finland and other supporters funded the work.
Which research steps follow
Where do we go from here? The researchers are convinced that the newly discovered mechanism of hydrogel formation is also relevant for other polymers and for their interactions with biological tissues.
Therefore, the team wants to chemically modify the polymers to see how this affects their properties and hydrogelation. It may be possible to specifically influence the gelation temperature as well as the strength and durability of the gel. From the modified materials, one could select those that are most suitable for use in biofabrication.
Funded by the Universitätsbund Würzburg, Ann-Christin Pöppler's team also wants to investigate whether the nanoparticles and thus also the hydrogel can be loaded with "guest molecules". This might be interesting for medical applications – if the gel dissolves at body temperature, it could release the active substances with which it was previously loaded. Applications in the form of implants, plasters or contact lenses are conceivable.