To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Surface chargeSurface charge is the electric charge present at an interface, for instance on the surface of a semiconductor material, or for example, on the surface of a protein in water. Additional recommended knowledgeThere are multiple factors generating surface charge. First of all, surface charge appears practically always on an object surface when it is placed into a fluid. All fluids contain ions, positive (cations) and negative (anions). These ions interact with the object surface. This interaction might lead to adsorption some of them on the surface. If number of adsorbed cations exceeds number of adsorbed anions, surface would gain total positive electric charge. This mechanism is important for colloids and other fluid based heterogeneous systems. There is another possible mechanism leading to surface charging in fluids. It is dissociation or Differential Solubility of the surface chemical group. The two ionic components of crystals like CaCO3, AgBr, BaSO4, and CaC2O4 always obey the bulk solubility equilibrium. E.g. for AgI Ksp = [Ag+][I-] The thermodynamic parameter used to describe charged surfaces is the surface potential.The surface potential,y0 , of an ionic crystal is related to the bulk concentration of a potential-determining ion by
i.e. AgI(s) can be precipitated by mixing aqueous solutions of AgNO3 and KI in any ratio. The equilibrium concentrations of [Ag+] and [I-] need not be equal. For mre details of the electric surfface charge description and its relation to the surface chemistry are given by Lyklema in "Fundamentals of Interface and Colloid Science" [1],
In conductors of uniform resistivity at equilibrium, there can be no free charges in the bulk, instead all the charge density is on the surface. In the case of conducting macroscopic bodies surface charge can be measured using electrostatic fieldmeters or voltmeters can also be used. In the case of colloids and similar heterogeneous fluid based systems, direct measurement of the surface charge is impossible due to small sizes of the objects. Instead, zeta potential measurement yields information for calculation surface charge. Another method is titration with appropriate surface active chemical. Some details are given in the book [2] The average surface charge density σ is given by
where q is the net amount of charge and A is the surface area of the interface. The measurement of surface charge density has applications in:
References
Categories: Colloidal chemistry | Condensed matter physics | Surface chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Surface_charge". A list of authors is available in Wikipedia. |





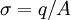 ,
,


