Chemists find new way to do light-driven reactions in solar energy quest
The more efficient method opens another possible pathway to harness sunlight for energy
Advertisement
Chemists have found a new, more efficient method to perform light-driven reactions, opening up another possible pathway to harness sunlight for energy. The journal Science is publishing the new method, which is based on plasmon - a special motion of electrons involved in the optical properties of metals.
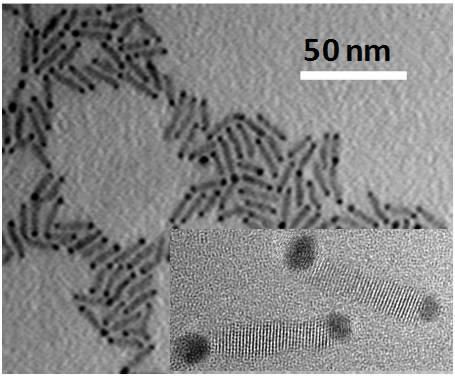
This is a transmission electron micrograph image of cadmium selenide nanorods with gold tips. Inset shows a high-resolution TEM image of two nanorods.
Micrograph images by James R. McBride
"We've discovered a new and unexpected way to use plasmonic metal that holds potential for use in solar energy conversion," says Tim Lian, professor of physical chemistry at Emory University and the lead author of the research. "We've shown that we can harvest the high energy electrons excited by light in plasmon and then use this energy to do chemistry."
Plasmon is a collective motion of free electrons in a metal that strongly absorbs and scatters light. One of the most vivid examples of surface plasmon can be seen in the intricate stained glass windows of some medieval cathedrals, an effect achieved through gold nano-particles that absorb and scatter visible light. Plasmon is highly tunable: Varying the size and shape of the gold nano-particles in the glass controls the color of the light emitted.
During photocatalysis, a metal absorbs light strongly, rapidly exciting a lot of electrons. "Imagine electrons sloshing up and down in the metal," Lian says. "Once you excite them at this level, they crash right down. All the energy is released as heat really fast - in picoseconds."
The researchers wanted to find a way to capture the energy in the excited electrons before it was released as heat and then use hot electrons to fuel reactions.
Through experimentation, they found that coupling nano-rods of cadmium selenide, a semi-conductor, to a plasmonic gold nanoparticle tip allowed the excited electrons in the gold to escape into the semi-conductor material.
Instead of using heat to do chemistry, this new process uses metals and light to do photochemistry, opening a new, potentially more efficient, method for exploration.
The researchers also want to explore whether the method can drive light-driven water oxidation more efficiently. Using sunlight to split water to generate hydrogen is a major goal in the quest for affordable and sustainable solar energy.






























































