Artificial leaf harnesses sunlight for efficient fuel production
A new solar fuel generation system safely creates fuel from sunlight and water.
Advertisement
Generating and storing renewable energy, such as solar or wind power, is a key barrier to a clean-energy economy. Over the past five years, researchers at Joint Center for artificial photosynthesis (JCAP) have made major advances toward this goal, and they now report the development of the first complete, efficient, safe, integrated solar-driven system for splitting water to create hydrogen fuels.
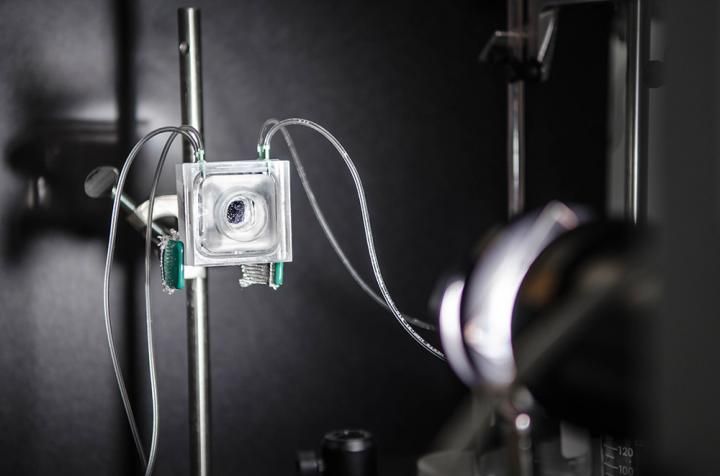
A highly efficient photoelectrochemical (PEC) device uses the power of the sun to split water into hydrogen and oxygen. The stand-alone prototype includes two chambers separated by a semi-permeable membrane that allows collection of both gas products.
Lance Hayashida/Caltech
The new solar fuel generation system, or artificial leaf was created by researchers in the laboratories of Caltech's Nate Lewis, George L. Argyros Professor and professor of chemistry and Harry Atwater, director of JCAP and Howard Hughes Professor of Applied Physics and Materials Science.
The new system consists of three main components: two electrodes - one photoanode and one photocathode - and a membrane. The photoanode uses sunlight to oxidize water molecules, generating protons and electrons as well as oxygen gas. The photocathode recombines the protons and electrons to form hydrogen gas. A key part of the JCAP design is the plastic membrane, which keeps the oxygen and hydrogen gases separate. The membrane lets the hydrogen fuel be separately collected under pressure and safely pushed into a pipeline.
Semiconductors such as silicon or gallium arsenide absorb light efficiently and are therefore used in solar panels. However, these materials also oxidize on the surface when exposed to water, so cannot be used to directly generate fuel. A major advance that allowed the integrated system to be developed was previous work in Lewis's laboratory, which showed that adding a nanometers-thick layer of titanium dioxide (TiO2 onto the electrodes could prevent them from corroding while still allowing light and electrons to pass through. The new complete solar fuel generation system developed by Lewis and colleagues uses such a TiO2 layer to effectively prevent corrosion and improve the stability of a gallium arsenide-based photoelectrode.
Another key advance is the use of active, inexpensive catalysts for fuel production. The photoanode requires a catalyst to drive the essential water-splitting reaction. Rare and expensive metals such as platinum can serve as effective catalysts, but in its work the team discovered that it could create a much cheaper, active catalyst by adding nickel to the surface of the TiO2. The photoanode was grown onto a photocathode, which also contains a highly active, inexpensive, nickel-molybdenum catalyst, to create a fully integrated single material that serves as a complete solar-driven water-splitting system.
A critical component that contributes to the efficiency and safety of the new system is the special plastic membrane that separates the gases, while still allowing the ions to flow seamlessly to complete the electrical circuit in the cell. The demonstration system is approximately one square centimeter in area, converts 10 percent of the energy in sunlight into stored energy in the chemical fuel, and can operate for more than 40 hours continuously.
"This new system shatters all of the combined safety, performance, and stability records for artificial leaf technology by factors of 5 to 10 or more," Lewis says.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.