Rock 'n' Roll Down the Tubes
Metal-filled fullerenes are "hopping" inside of carbon nanotubes
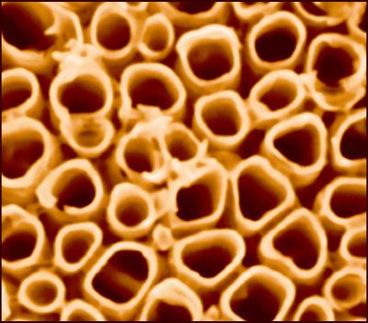
The technical world keeps getting smaller. Eventually the miniaturization of chips will reach dimensions at which individual atoms will have to be manipulated. As components of a quantum computer or nanoelectronics of the future, self-assembled molecular arrangements that look like nanoscopic pea pods-carbon nanotubes filled with spherical fullerene molecules-are seen as promising candidates. The fullerenes themselves can accommodate metal atoms in their cavities. However, before useful electronic devices can be made, the properties of peapods must be studied to evaluate the potential of such structures. A research team has now observed that such "peas" perform strange hopping motions in their "pods".
Andrei Khlobystov and Andrew Briggs of Oxford University, and John Dennis of Queen Mary College, University of London and their coworkers studied the motion of fullerenes made of 82 carbon atoms (spherical cage-like molecules) each filled with a cerium atom. Within a crystal, these molecular cages rotate freely. What happens when the peas are put into "pods", or carbon nanotubes? With a high-resolution electron microscope, the fullerenes are easy to spot within nanotubes, and the peas can be seen to move laterally. If the pods are only partially filled, the peas hop back and forth abruptly, irregularly, and independently of one another. In completely filled pods the motion of the tightly packed peas is slower and more continuous-and the whole row moves together!
Because the cerium atom in each fullerene can be visualized with the electron microscope, the rotational motion of the peas can also be understood. This is possible because the cerium atoms are not set at the center of the fullerene, but are "stuck" fast to one side of the "shell". Owing to the intermolecular interactions between the fullerenes and the nanotube, there is a preferred orientation of the fullerenes relative to the axis of the tube. Each fullerene is also affected by its two immediate neighbors. The free rotation present in a crystal, in which each fullerene has 12 immediate neighbors, has disappeared; the fullerenes now jump from one orientation to the next, then rest, and throw themselves into yet another position, quickly, abruptly, and irregularly. If individual pea pods are packed into bundles, the discontinuous rotation gets faster. This occurs because of the additional interactions of the fullerenes with neighbors in other tubes in the bundle. In structures with more complex symmetry the rotation is clearly less restricted than in isolated nanotubes. The state of the bundle is thus something of an intermediate between the three-dimensional crystal lattice and the quasi-one-dimensional state in the isolated nanotubes.
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.