To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Dissociation constant
In chemistry and biochemistry, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is usually denoted Kd and is the inverse of the affinity constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction in which a complex AxBy breaks down into x A subunits and y B subunits, the dissociation constant is defined where [A], [B], and [AxBy] are the concentrations of A, B, and the complex AxBy, respectively.
Product highlight
Protein-Ligand bindingThe dissociation constant is commonly used to describe the affinity between a ligand (L) (such as a drug) and a protein (P) i.e. how tightly a ligand binds to a particular protein. Ligand-protein affinities are influenced by non-covalent intermolecular interactions between the two molecules such as hydrogen bonding, electrostatic interactions , hydrophobic and Van der Waals forces. The formation of a ligand-protein complex (C) can be described by a two-state process the corresponding dissociation constant is defined where [P], [L] and [C] represent the concentrations of the protein, ligand and complex, respectively. The dissociation constant has molar units (M), which correspond to the concentration of ligand [L] at which the binding site on a particular protein is half occupied, i.e. the concentration of ligand, at which the concentration of protein with ligand bound [C], equals the concentration of protein with no ligand bound [P]. The smaller the dissociation constant, the more tightly bound the ligand is, or the higher the affinity between ligand and protein. For example, a ligand with a nanomolar (nM) dissociation constant binds more tightly to a particular protein than a ligand with a micromolar (μM) dissociation constant. Sub-nanomolar dissociation constants as a result of non-covalent binding interactions between two molecules are rare. Nevertheless, there are some important exceptions. Biotin and avidin bind with a dissociation constant of roughly 10 − 15 M = 1 fM = 0.000001 nM.[1] While ribonuclease inhibitor proteins may also bind to ribonuclease with a similar 10 − 15 M affinity.[2] The dissociation constant for a particular ligand-protein interaction can change significantly with solution conditions (e.g. temperature, pH and salt concentration). The effect of different solution conditions is to effectively modify the strength of any intermolecular interactions holding a particular ligand-protein complex together. Drugs can produce harmful side effects through interactions with proteins for which they were not meant to or designed to interact. Therefore much pharmaceutical research is aimed at designing drugs that bind to only their target proteins with high affinity (typically 0.1-10 nM) or at improving the affinity between a particular drug and its in-vivo protein target. Another notationA dissociation constant Ka is sometimes expressed by its pKa, which is defined as:
These pKa's are mainly used for covalent dissociations (i.e., reactions in which chemical bonds are made or broken) since such dissociation constants can vary greatly. Dissociation constant of waterAs a frequently used special case, the dissociation constant of water is often expressed as Kw: Kw = [H + ][OH − ] (The concentration of water The value of Kw varies with temperature, as shown in the table below. This variation must be taken into account when making precise measurements of quantities such as pH.
Acid base reactionsFor the deprotonation of acids, K is known as Ka, the acid dissociation constant. Stronger acids, for example sulfuric or phosphoric acid, have larger dissociation constants; weaker acids, like acetic acid, have smaller dissociation constants. A molecule can have several acid dissociation constants. In this regard, that is depending on the number of the protons they can give up, we define monoprotic, diprotic and triprotic acids. The first (e.g. acetic acid or ammonium) have only one dissociable group, the second (carbonic acid, bicarbonate, glycine) have two dissociable groups and the third (e.g. phosphoric acid) have three dissociable groups. In the case of multiple pK values they are designated by indices: pK1, pK2, pK3 and so on. For amino acids, the pK1 constant refers to its carboxyl (-COOH) group, pK2 refers to its amino (-NH3) group and the pK3 is the pK value of its side chain.
References
See also |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Dissociation_constant". A list of authors is available in Wikipedia. |


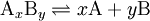
![K_{d} = \frac{[A]^x \times [B]^y}{[A_x B_y]}](images/math/f/a/7/fa734f7c67752b156fc481a917e07f30.png)



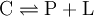
![K_{d} = \frac{\left[ \mathrm{P} \right] \left[ \mathrm{L} \right]}{\left[ \mathrm{C} \right]}](images/math/0/2/d/02d9662e84613b8f2ab83ae3d30de36c.png)
![\left[ \mbox{H}_2\mbox{O} \right]](images/math/0/7/9/0790e5e6b5eab215ab1c4bcfe59ba835.png) is not included in the definition
of
is not included in the definition
of ![H_3 B \rightleftharpoons\ H ^ + + H_2 B ^ - \qquad K_1 = {[H ^ +] \cdot [H_2 B ^ -] \over [H_3 B]} \qquad pK_1 = - log K_1](images/math/c/3/d/c3d829b2b15e365a3fa324f3004cd577.png)
![H_2 B ^ - \rightleftharpoons\ H ^ + + H B ^ {-2} \qquad K_2 = {[H ^ +] \cdot [H B ^{-2}] \over [H_2 B^ -]} \qquad pK_2 = - log K_2](images/math/6/a/5/6a5ed60798b92de536cb223161396238.png)
![H B ^{-2} \rightleftharpoons\ H ^ + + B ^{-3} \qquad K_3 = {[H ^ +] \cdot [ B ^ {-3}] \over [H B ^ {-2}]} \qquad pK_3 = - log K_3](images/math/5/2/3/523fea83f75ad71ed89710b4c1458baf.png)


