To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Reaction quotientIn chemistry, reaction quotient is a quantitative measure of the extent of reaction, the relative proportion of products and reactants present in the reaction mixture at some instant of time.
Product highlightFor a chemical mixture with certain initial concentrations of reactants and products, it is useful to know if the reaction will shift to the right/in the forward direction (increasing the concentrations of the products) or if it will shift to the left/in the reverse direction (increasing the concentrations of the reactants). Given a general equilibrium expression such as
where A, B, C, and D are chemical species involved in this reaction and k, m, n, and p are the stoichiometric coefficients for the reaction, the reaction quotient, Q, is defined as [1]: where the { Ai } denotes the instantaneous activity[2] of the species A at a certain moment of time and so on for the other species. The reaction quotient is taken at a particular instant in time, not necessarily the moment when equilibrium is reached. The reaction quotient is directly related to Le Chatelier's Principle. For a reaction at chemical equilibrium, the equilibrium constant, K, may be defined as: where {A} is the activity of the species A when the mixture is at equilibrium, etc. By comparing the values of Q and K, one can determine whether the reaction will shift to the right, to the left, or if the concentrations will remain the same (equilibrium).
The relationship of reaction quotient Q with the instantaneous derivative of Gibbs energy (ΔG) and standard change of Gibbs energy (ΔG ΔG = ΔG See alsoReferences
|
|||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Reaction_quotient". A list of authors is available in Wikipedia. |





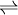 nC + pD ...
nC + pD ...