To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Ubbelohde viscometerA Ubbelohde type viscometer or suspended-level viscometer is a measuring instrument which uses a capillary based method of measuring viscosity [1]. It is recommended for higher viscosity cellulosic polymer solutions. The advantage of this instrument is that the values obtained are independent of the concentration. The device was invented by the German chemist Leo Ubbelohde (1877-1964). Product highlightASTM and other test methods are: ISO 3104, ISO 3105, ASTM D 445, ASTM D 446, IP 71, BS 188 [2] The Ubbelohde viscometer is closely related to the Ostwald viscometer. Both are u-shaped pieces of glassware with a reservoir on the right and a measuring bulb with a capillary on the left. A liquid is introduced and a pressure head forces this liquid from the bulb through the capillary to the reservoir. The time it takes for the liquid to pass through two calibrated marks is a measure for viscosity. The Ubbelohde device has a third arm extending from the end of the capillary and open to the atmosphere. In this way the pressure head only depends on a fixed height and no longer on the total volume of liquid.
The determination of viscosity is based on Poiseuille's law:
where t is the time it takes for a volume V to elute. The ratio dv/dt depends on R as the capillary radius, on the average applied pressure P, on its length L and on the viscosity η. The average pressure head is given by:
Usually the viscosity of a liquid is compared to a liquid with an analyte for example a polymer dissolved in it. The relative viscosity is given by:
This specific viscosity is related to the concentration of the analyte through the Intrinsic viscosity [η] by the power series:
or
where ηsp/c is called the viscosity number. The intrinsic viscosity can be determined experimentally by measuring the viscosity number as function of concentration as the Y-axis intercept.
ReferencesCategories: Laboratory glassware | Polymer chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Ubbelohde_viscometer". A list of authors is available in Wikipedia. |





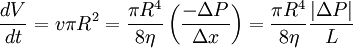
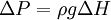
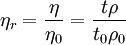 .
.
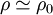
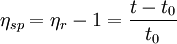 .
.
![\eta_{sp} = [\eta] c + k [\eta]^2 c^2 + ....... \,](images/math/2/e/0/2e0940c19a4279408a7cef71afea7694.png)
![\frac{\eta_{sp}}{c} = [\eta] + k [\eta]^2 c + ....... \,](images/math/3/a/8/3a899d7424bf4410b7c8d1f9d3cd9948.png)


