New microscopy method resolves fluorescent molecules with resolution at the nanometer scale
Advertisement
Scientists working with Stefan Hell at the Max Planck Institute (MPI) for Biophysical Chemistry in Göttingen and the Heidelberg-based MPI for Medical Research have developed another light microscopy method, called MINSTED, which resolves fluorescently labeled details with molecular sharpness. With MINSTED, Nobel laureate Hell has come full circle.
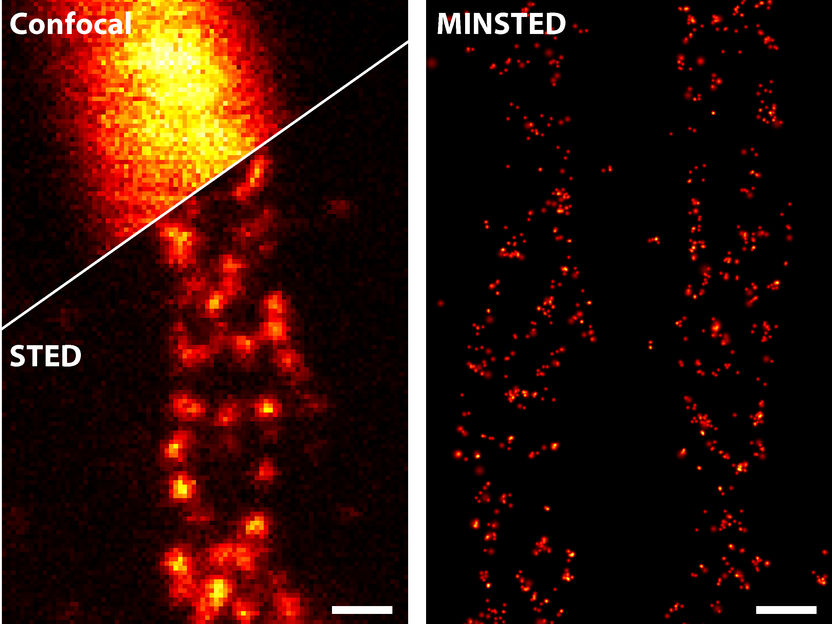
A comparison of different light microscopy techniques: STED (bottom left) improves the sharpness of detail drastically compared to conventional confocal microscopy (top left). MINSTED (right) achieves a resolution that is yet ten times higher.
© Michael Weber / Max-Planck-Institut für biophysikalische Chemie
“A good 20 years ago, we fundamentally broke the diffraction resolution limit of fluorescence microscopy with STED. Until then, that was considered impossible,” says Hell. “Back then we dreamed: With STED we want to become so good that one day we will be able to separate individual molecules that are only a few nanometers apart. Now we've succeeded.” At that time, the STED principle amounted to a revolution in light microscopy. For this conceptual leap and subsequent developments, Hell received the Nobel Prize in Chemistry in 2014.
In its original version, STED microscopy achieved a resolution of 20 to 30 nanometers (millionths of a millimeter) and was thus around ten times sharper than the light microscopes available up to that point. In 2016, Hell and his coworkers were able to further increase the resolution tenfold through a new method called MINFLUX nanoscopy. In that new approach, they combined an element from the STED principle with one from another light microscopic technique, PALM/STORM, and thus achieved a resolution of just a few nanometers for the first time. MINFLUX can truly make fluorescent molecules visible on molecular scales – it does not get any sharper.
A new family of nanometer resolution microscopes
Hell was convinced that MINFLUX would not remain the only molecular resolution method, but rather would represent the first member of a new family of techniques with this level of detail. With MINSTED, he and his coworkers are now showing this to be true. As the name suggests, MINSTED relies on the original STED principle even more than MINFLUX. Elaborating on this, Michael Weber, a PhD student in Hell’s laboratory and one of the developers of MINSTED, says: “This has advantages. Like MINFLUX, it achieves molecular resolution, but the background noise is lower. In addition, the resolution can now be adjusted almost continuously from 200 nanometers down to the molecular size – 1 nanometer.”
With MINSTED, Hell is building on his breakthrough with STED more than 20 years ago and is extracting the full potential from this concept. “Microscopy on the molecular scale is here to stay. It is to be expected that MINSTED and MINFLUX will become widely used in the life sciences,” says the physicist.
Switching the fluorescence of molecules on and off
STED achieved what was previously unattainable, separately detecting molecules less than 200 nanometers apart, with a trick: Neighboring fluorescent features or molecules are switched on and off one after the other. To do this, a laser beam that excites the molecules is immediately followed by a second one, the so-called STED beam, which prevents the molecules from fluorescing. The STED beam, however, has a ‘hole’ in the middle. In other words, it is donut-shaped. Only the molecules in the middle of this donut beam can fluoresce. Thus, one always knows where the emitting molecules are. In practice, STED does not achieve molecular resolution because the donut-shaped fluorescence inhibition beam cannot be made so strong that only a single molecule can fit into the hole.
For this reason in MINSTED, the fluorescent molecules are initially isolated by randomly switching them on through an independent photochemical switching process, rather than by the donut beam itself. The fluorescence-preventing STED donut beam is then used to locate the fluorescent molecules individually. Its hole serves as a reference point. “If the hole coincides with the molecule, the molecule glows most strongly and you can figure out precisely where it is, because the exact position of the STED donut beam is always known,” says Marcel Leutenegger, a postdoctoral researcher in Hell's department. “That’s why we gradually approach the molecules in a targeted fashion with the donut beam and can thus locate the fluorescent molecules with an accuracy of 1 to 3 nanometers, that is, the size of the molecules. In connection with the photochemical on and off switching, the resolution becomes molecular-scale.”

































































