Nanotechnology: Study shows urgent need for reference materials
Two new developments could bring decisive progress
Advertisement
Whether in electronic components, innovative medicines, or modern environmental analysis, nanomaterials are found in many technologies of the future. Precise reference standards are needed to ensure that they can be used safely and reliably. However, there are significant gaps in this area, as a recent study by the Federal Institute for Materials Research and Testing (BAM) and the Metrology Research Centre Canada shows. Two new developments could bring decisive progress.
Reference materials are high-quality, comprehensively characterized samples that laboratories can use to control and calibrate their instruments and develop new measuring methods or establish them in their laboratories. Reference materials ensure that measurements are comparable, and results are reliable. "Without reliable reference materials, we cannot guarantee that nanomaterials are safe – neither for humans nor for the environment," emphasizes Ute Resch-Genger from the Federal Institute for Materials Research and Testing (BAM).
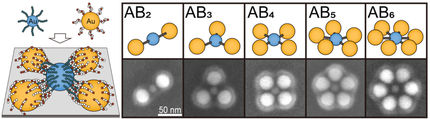
Although some nano reference materials already exist, they do not cover many important properties such as exact shape, size distribution, composition, surface chemistry, and particle number concentration. This is particularly critical in medicine, where nanoparticles are used in vaccines and cancer therapies and for the risk assessment of nanomaterials.
New reference materials for greater safety
Now, two new developments give cause for hope: for the first time, scientists at BAM and the Metrology Research Centre have developed two new nano reference materials—iron oxide nanocubes and lipid-based nanoparticles.
Iron oxide nanoparticles are used, for example, in imaging techniques such as magnetic resonance imaging (MRI). Since they are not only spherical but can also have different shapes or a wide size distribution, reference materials in cube form have been developed for the first time.
Lipid-based nanoparticles play an important role as carrier systems for drugs, for example in vaccines or cancer therapies. They help to place active ingredients precisely in the body and reduce side effects. The fact that the first reference materials for such nanomedicines are now available is an important step towards the safe use of nanomaterials in medicine and the life sciences.
More need for research
Despite this progress, there is still a considerable need for action. The authors of the study emphasize the need to develop further reference materials—for example, with known surface chemistry, as is currently being pursued in the European metrology project SMURFnano coordinated by BAM (23NRM02). In addition, materials are needed that can represent several properties simultaneously and can be used under practical conditions. It is also particularly important to make characterization data on nanomaterials available in open accessible databases. This will speed up the development and characterization of new nanomaterials and their safe use.