Liquid Fuels from Carbon Dioxide
Electrocatalyst converts CO2 into multicarbon products
Advertisement
A new electrocatalyst called a-CuTi@Cu converts carbon dioxide (CO2 ) into liquid fuels. As reported by a team of Chinese researchers in the journal Angewandte Chemie, active copper centered on an amorphous copper/titanium alloy produces ethanol, acetone, and n-butanol with high efficiency.
© Wiley-VCH
Most of our global energy demands are still being met by burning fossil fuels, which contributes to the greenhouse effect through the release of CO2 . To reduce global warming, we must look for opportunities to use CO2 as a raw material for basic chemicals. Through electrocatalytic conversion of CO2 using renewable energy, a climate-neutral, artificial carbon cycle could be established. Excess energy produced by photovoltaics and wind energy could be stored through the electrocatalytic production of fuels from CO2. These could then be burned as needed. Conversion into liquid fuels would be advantageous because they have high energy density and are safe to store and transport. However, the electrocatalytic formation of products with two or more carbon atoms (C2+) is very challenging.
A team from Foshan University (Foshan, Guangdong), the University of Science and Technology of China (Hefei, Anhui), and Xi’an Shiyou University (Xi’an, Shaanxi), led by Fei Hu, Tingting Kong, Jun Jiang, and Yujie Xiong has now developed a novel electrocatalyst that efficiently converts CO2 to liquid fuels with multiple carbon atoms (C2–4). The primary products are ethanol, acetone, and n-butanol.
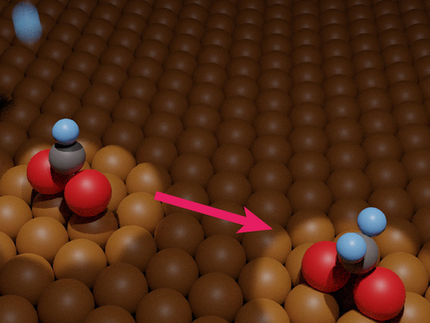
To make the electrocatalyst, thin ribbons of a copper/titanium alloy are etched with hydrofluoric acid to remove the titanium from the surface. This results in a material named a-CuTi@Cu, with a porous copper surface on an amorphous CuTi alloy. It has catalytically active copper centers with remarkably high activity, selectivity, and stability for the reduction of CO2 to C2+ products (total faradaic efficiency of about 49 % at 0.8 V vs. reversible hydrogen electrode for C2–4, and it is stable for at least three months). In contrast, pure copper foil produces C1 products but hardly any C2+ products.
The reaction involves a multistep electron-transfer process via various intermediates. In the new electrocatalyst, the inactive titanium atoms below the surface actually play an important role; they increase the electron density of the Cu atoms on the surface. This stabilizes the adsorption of *CO, the key intermediate in the formation of multicarbon products, allows for high coverage of the surface with *CO, and lowers the energy barrier for di- and trimerization of the *CO as new carbon–carbon bonds are formed.