Nanoneedles formed on an electrocatalyst improve hydrogen production
Advertisement
The low-cost, efficient production of hydrogen is an important step toward developing alternative, clean energy sources. electrochemical water splitting, which splits water into its hydrogen and oxygen elements using an electrocatalyst, is a viable option for producing hydrogen. Conventionally, catalysts have been based on costly elements such as platinum, which makes it difficult to apply this technology on a widespread, commercial scale.
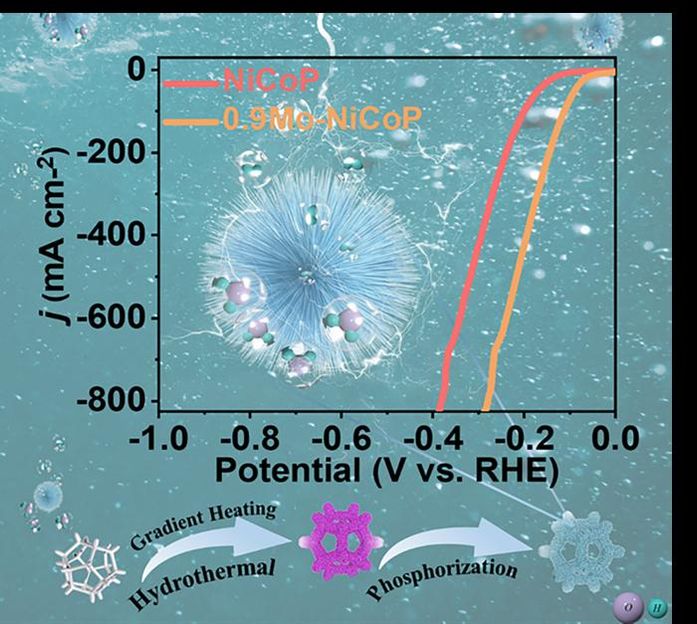
This diagram shows the nanoneedle structure of the electrocatalyst that is molybdenum-doped nickel-cobalt phosphide. The graph demonstrates the polarization curve, which demonstrates the improvement in the electrocatalyst when molybdenum is added to it. The graphic on the bottom also illustrates the gradient heating hydrothermal process to produce the electrocatalyst.
Nano Research, Tsinghua University Press
In a recently published paper, researchers demonstrated how adding molybdenum to a nickel-cobalt phosphide catalyst and synthesizing it with a gradient hydrothermal process, in which the catalyst is heated to 100 degrees, 150 degrees, and then 180 degrees Celsius over 10 hours, created a unique microstructure that improved the performance of the catalyst, resulting in hydrogen production that could be more applicable to large-scale hydrogen production.
“The innovative combination of gradient hydrothermal and phosphidation processes forms a microsphere structure,” said Yufeng Zhao, a professor at the College of Sciences & Institute for Sustainable Energy at Shanghai University in Shanghai, China. “These nanoparticles with a diameter of approximately 5 to 10 nanometers form nanoneedles, which subsequently self-assemble into a spherical structure. The nanoneedles offer abundant active sites for efficient electron transfer and the presence of small-sized particles and micro-scale roughness enhances the release of hydrogen bubbles.”
To create this unique microstructure, researchers employed a technique called element doping. Element doping is the intentional addition of impurities to a catalyst to improve its activity. In this study, molybdenum (Mo) was added to the bimetallic nickel-cobalt (Ni-Co) phosphide (P). Ni-Co phosphides already have exceptional electrocatalytic performance because of the way the cobalt and nickel ions interact. After adding the molybdenum and then using a gradient hydrothermal process, the Mo-doped Ni-CoP was deposited onto a nickel foam. After this process, the unique microstructure of nanoneedles formed on the phosphide.
“Trace molybdenum doping optimizes the electronic structure and increases the number of electroactive sites,” said Zhao. The Mo-doped Ni-CoP catalyst was tested for reliability, stability, and performance. Its density remained nearly constant after 100 hours and its structure was well-maintained, thanks in part to the unique structure of the nanoneedles, which prevent the catalyst from collapsing as hydrogen accumulates. Calculations also showed that the phosphide catalyst was exceptionally efficient.
Looking ahead, researchers hope to test the performance of the reaction in different solutions, such as acidic and neutral solutions. Future studies will also look at alternatives to nickel foam, such as titanium mesh, that can operate across the pH range. “In future work, we recommend exploring the application of the catalyst in the oxidation-assisted hydrogen production of small molecules, such as urea. This approach would reduce the overpotential of water electrolysis and mitigate environmental pollution caused by urea wastewater,” said Zhao.

































































