Chemists develop molecule for important step towards artificial photosynthesis
Advertisement
A research team at the University of Basel has developed a new molecule that is modeled on photosynthesis in plants: Under the influence of light, it simultaneously stores two positive and two negative charges. The aim is to convert sunlight into CO₂-neutral fuels.
Plants use the energy of sunlight to convert CO₂ into energy-rich sugar molecules. This process is called photosynthesis and is the basis of practically all life: animals and humans can "burn" the carbohydrates produced in this way and use the energy stored in them. This produces carbon dioxide again, closing the cycle.
This model could also be the key to environmentally friendly fuels: Researchers are working on imitating natural photosynthesis and using sunlight to produce energy-rich compounds: so-called solar fuels such as hydrogen, methanol or synthetic gasoline. When they are burned, only as much carbon dioxide is produced as was needed to produce the fuels. They would therefore be CO₂-neutral.
Molecule with a special structure
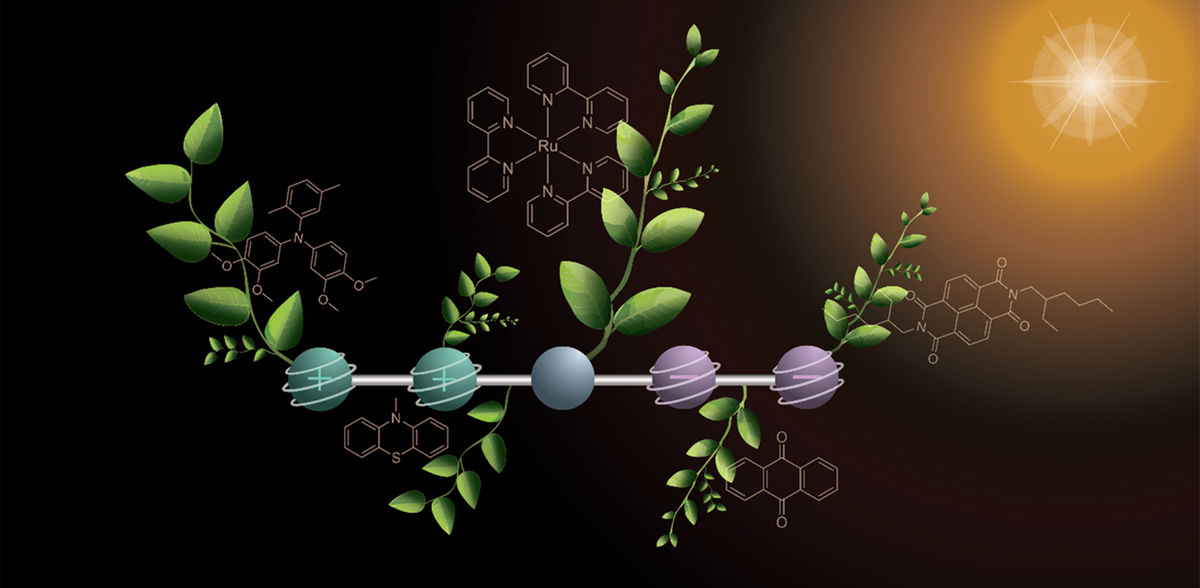
Prof. Dr. Oliver Wenger and his doctoral student Mathis Brändlin have now reported an important intermediate step towards this vision of artificial photosynthesis in the journal "Nature Chemistry": they have developed a special molecule that can store four charges simultaneously when exposed to light - two positive and two negative charges.
The temporary storage of several charges is an important prerequisite for converting sunlight into chemical energy: The charges can be used to drive reactions - for example, to split water into hydrogen and oxygen.
The molecule consists of five parts, which are linked in a row and each fulfill a specific task. On one side of the molecule there are two parts that release electrons and become positively charged in the process. Two on the other side accept the electrons and thus become negatively charged. In the middle, the chemists placed a building block that captures sunlight and starts the reaction (electron transfer).
Two steps with light
To generate the four charges, the researchers proceeded step by step with two flashes of light. The first flash of light hits the molecule and triggers a reaction in which a positive and a negative charge are created. These charges move outwards to the opposite ends of the molecule. The second flash of light triggers the same reaction again, so that the molecule now contains two positive and two negative charges.
Works with weak light
"This step-by-step excitation makes it possible to use much weaker light. We are already close to the strength of sunlight," explains Brändlin. In previous research work, extremely strong laser light was required, which was a far cry from the vision of artificial photosynthesis. "In addition, the charges in the molecule remain stable long enough to be used for further chemical reactions."
The new molecule has not yet created a functioning artificial photosynthesis system. "But we have identified and realized an important piece of the puzzle," says Oliver Wenger. The new findings from the study contribute to a better understanding of the electron transfers that are central to artificial photosynthesis. "We hope to contribute to new perspectives for a sustainable energy future," says Wenger.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.