Tiny switches, big effect
Mechanism of photoswitches decoded - with potential for medicine, materials and electronics
Advertisement
An inter-university team from TU Wien and the University of Vienna has made important progress in understanding so-called photoswitches. These tiny molecular "light switches" change their structure when they are irradiated with light - similar to a switch that changes between "on" and "off". Applied to chemistry, this means that the molecule changes from its stretched to its bent form, thereby changing its chemical properties. The ability to switch molecules in a targeted manner opens up prospects for applications in medicine, materials science and data storage. The research results have now been published in the journal Angewandte Chemie.
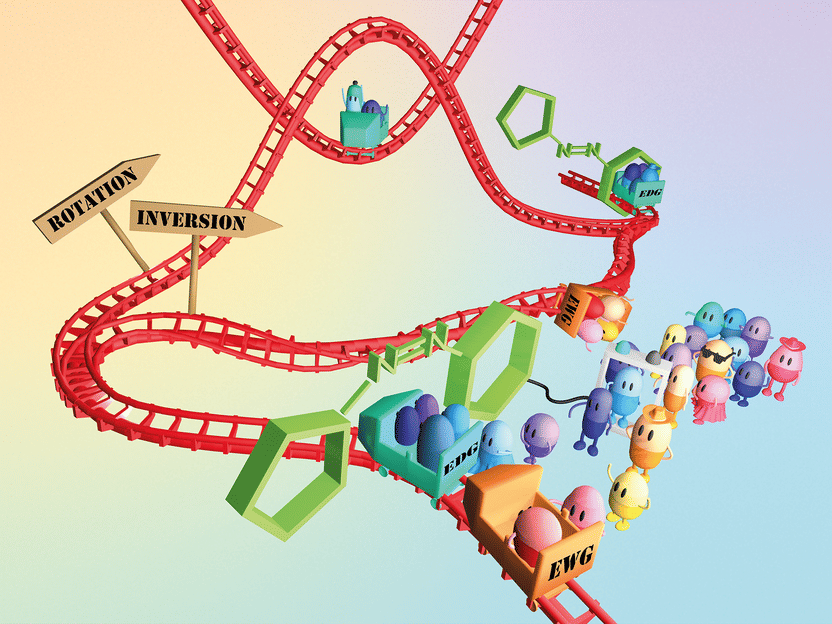
You can think of photo switches as a roller coaster with two switches: the left side goes fast, the right side goes slow.
Copyright: Maximilian Wutscher
Understanding and predicting molecular switches
For a long time, it was unclear why certain molecules behave in one way or another. Now the team has been able to show that even the smallest chemical changes - so-called substituents - are decisive in determining whether a photoswitch remains active for only seconds or remains stable over a longer period of time. The team focused on the arylazopyrazole class of substances, which has been investigated as potential photoswitches for some time.
Marko Mihovilovic, Dean of the Faculty of Technical Chemistry at TU Wien, compares the underlying process to a rollercoaster ride: "You can imagine it like a rollercoaster with two switches: If I turn left, the molecule switches quickly, if I turn right, it switches slowly. But I have to understand this process in order to be able to switch in a targeted manner."
The team succeeded in understanding exactly how this works by linking the theoretical model from the University of Vienna and the experimental data from TU Wien. Only the interaction of both approaches made it possible to precisely understand and predict the switching times.
New perspectives through targeted control
With the new knowledge, it has now been possible for the first time to "tailor" the lifespan of such photoswitches. This is a decisive step towards applications in which molecules can be activated by light in a controlled manner. In photopharmacology in particular - i.e. drugs that only become effective at the desired location in the body through light - this could help to reduce side effects and control treatments more precisely. But new possibilities are also opening up in electronics, where extremely short switching times are required, or in materials research, where materials are supposed to change at the touch of a button.
"We are molecular engineers - we assemble molecules so that they have the desired properties," says Mihovilovic. "If we understand how the molecular switching processes work, we can control effects ranging from the microscopic to the macroscopically measurable."
Theory and experiment complement each other
The theoretical side also played a decisive role. "Only with calculations can we understand why some molecules switch quickly and others slowly - and how these properties can be predicted," explains Leticia González from the Institute of Theoretical Chemistry at the University of Vienna. "This makes it possible to specifically develop new molecules with tailored switching properties without having to rely on pure trial and error."
Moreover, the knowledge is not limited to the field of photopharmacology, even though this is the focus of the research team. This is because the way it works is the same, regardless of whether it is about molecules in materials or pharmaceuticals. The work was carried out in cooperation between the Institute of Applied Synthetic Chemistry at TU Wien and the Institute of Theoretical Chemistry at the University of Vienna. The project was funded by the Austrian Science Fund (FWF).
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.