Prussian Blue breaks out of its cubic mold after 300 years
Advertisement
For the first time in more than three centuries, Prussian Blue – long confined to its rigid cubic shape – has been transformed into an octahedral structure. A research team led by Prof. Changshin Jo (Department of Battery Engineering & Department of Chemical Engineering, POSTECH), Prof. Sangmin Lee (Department of Chemical Engineering, POSTECH), and Ph.D candidate Seunghye Jang (Department of Battery Engineering, POSTECH) has successfully synthesized this new morphology by replacing water with a specialized solvent, glycerol, during the crystal growth process. Their findings were published online in Advanced Functional Materials, an international journal in materials and energy research.
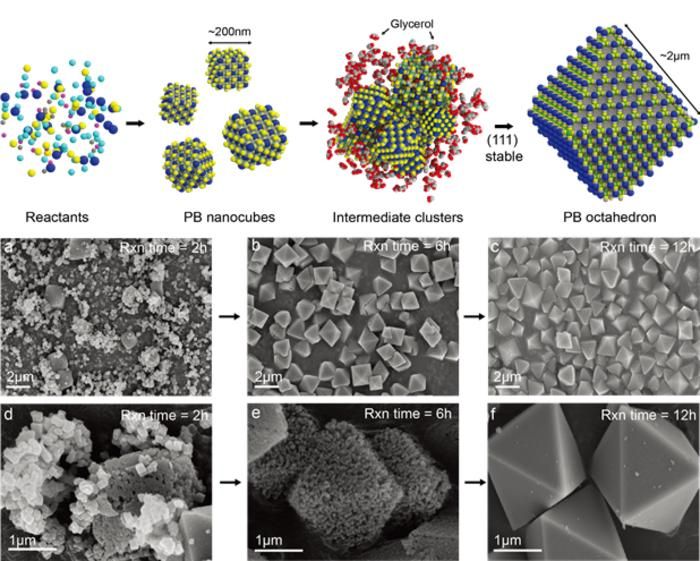
Schematic illustration of the particle formation mechanism of glycerol-based octahedral Prussian Blue
POSTECH
Accidentally discovered around 1700s, Prussian Blue possesses a hollow three-dimensional framework that allows ions to move in and out with ease. These unique properties have enabled its use across diverse fields, from sodium-ion battery electrodes to radioactive cesium removal, catalysis, and environmental remediation.
However, until now, its morphology had been limited. When synthesized in water, the reaction proceeds too quickly, making it difficult to control particle growth and producing only cubic particles. This constraint has prevented scientists from exploring shape-dependent properties or unlocking new applications.
The POSTECH researchers found the solution in the solvent. By using viscous glycerol instead of water, they were able to slow down crystal growth. In this glycerol medium, small cubic particles initially nucleated, then repeatedly dissolved and recrystallized, self-assembling into octahedra structures. In effect, these tiny cubes stacked and transformed into gem-like eight-faced structures.
When tested as an electrode material in sodium-ion hybrid capacitors, the octahedral Prussian Blue demonstrated remarkable advantages. Its higher surface area enhanced electrochemical reactivity, while long-term charge–discharge cycling test confirmed stable performance. Simply changing the crystal shape resulted in significant performance improvements. The research also involved Dr. Carsten Korte of Forschungszentrum Jülich (Germany), who contributed to the structural analysis.
This study is the first to demonstrate that specific solvents can control both the growth rate and the orientation of Prussian Blue crystals. Beyond glycerol, the team anticipates that other organic solvents may enable the design of previously unprecedented crystal morphologies.
Prof. Jo stated, “The significance of this research lies not only in successfully creating a new morphology of Prussian Blue, but also in establishing the fundamental principles that enable us to observe and control its growth process. With the capability to design diverse morphologies, we anticipate a substantial expansion of applications, ranging from advanced energy storage systems to environmental purification technologies.”
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.































































