Lead-acid battery inspired catalyst design
Pb doped RuIrOx for efficient and durable acidic oxygen evolution in proton exchange membrane electrolyzers at 3 A/cm2
Advertisement
A n international research team from the Songshan Lake Materials Laboratory (SLAB), the Institute of Physics at the Chinese Academy of Sciences, and the International Iberian Nanotechnology Laboratory have developed a novel lead-doped ruthenium-iridium oxide (RuIrPbOₓ) catalyst that exhibits outstanding stability and efficiency for oxygen evolution reactions (OER) in proton exchange membrane water electrolyzers (PEMWEs) operating at high current densities of 3 A/cm². This work addresses longstanding challenges in catalyst durability and performance under acidic, harsh conditions, paving the way for more reliable and cost-effective hydrogen production technologies.
Hydrogen production via proton exchange membrane water electrolysis is a well-known and promising clean energy solution. Central to this technology are catalysts that facilitate the oxygen evolution reaction (OER) under highly acidic conditions. Currently, the state-of-the-art catalysts, mainly based on ruthenium and iridium oxides, have demonstrated high activity but suffer from significant durability issues when operated at high current densities. Their structural degradation, including lattice oxygen participation and metal leaching, limits the operational lifespan and hinders practical deployment. Moreover, balancing activity, stability, and cost remains a persistent challenge, largely due to the corrosive nature of the environment and the scarcity of noble metals.
Currently, most PEM electrolyzers rely on iridium-based catalysts because IrO₂ can withstand the corrosive acidic environment. Yet iridium is one of the rarest and most expensive elements on Earth, and its global supply chain is vulnerable to geopolitical fluctuations. Ruthenium, while more abundant and active, suffers from rapid dissolution under high voltage, forming soluble RuO₄ species that lead to fast degradation. Researchers worldwide have sought ways to stabilize Ru–Ir oxide catalysts or replace iridium altogether — but maintaining both high activity and long-term durability under industrial current densities has remained a formidable challenge.
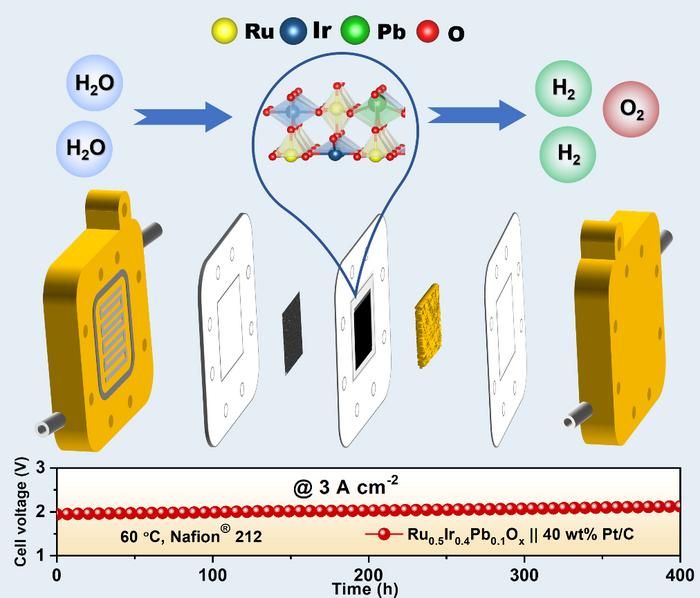
RuIrPbOx as PEMWE anode catalysts.
Fei Lin and Lifeng Liu from Songshan Lake Materials Laboratory.
The Solution
Drawing inspiration from the robust chemistry of lead-acid batteries, the researchers doped lead atoms into the Ru-Ir oxide lattice through a simple sol-gel process. The lead atoms act as electronic stabilizers, tuning the local structure of ruthenium sites and preventing their dissolution, a key failure mode in conventional catalysts.
Advanced characterization revealed that the optimal Pb doping suppresses the destructive participation of lattice oxygen in the oxygen-evolution reaction, preserving the catalyst’s integrity. Density functional theory (DFT) calculations further confirmed that Pb doping reduces Ru–O covalency and increases Ru dissolution energy, effectively preventing Ru leaching. When integrated into a full PEM electrolyzer, this catalyst enabled water electrolysis at 1.96 V @ 3 A cm⁻², surpassing commercial IrO₂ and RuO₂ electrodes while using significantly less precious metal. The total platinum-group-metal loading (Pt + Ru + Ir) was reduced to 0.17 mg W⁻¹, approaching the U.S. DOE 2026 target and outperforming the EU Clean Hydrogen 2030 target for PEMWE.
The Future
Future work will focus on scaling up electrode fabrication and optimizing catalyst integration into large-area PEM stacks. The team also plans to explore alternative dopants and nanostructuring strategies to further cut precious-metal consumption and enhance performance at even higher current densities.
These studies aim to accelerate the industrial deployment of PEM water electrolyzers powered by renewable energy – an essential step toward a global green-hydrogen economy.
The Impact
This discovery demonstrates a practical route to producing efficient and durable catalysts for acidic water electrolysis, overcoming one of the major bottlenecks to large-scale green-hydrogen production. By combining cost reduction, high current-density capability, and exceptional stability, the lead-modified Ru–Ir oxide catalyst sets a new benchmark for next-generation electrolyzer technology.
The work not only advances fundamental understanding of stability mechanisms in precious-metal oxides but also provides a blueprint for designing robust electrocatalysts for industrial renewable-energy systems.
Original publication
Jingwei Wang, Kaiyang Xu, Zhipeng Yu, Hang Cui, Haoliang Huang, Chenyue Zhang, Run Ran, Liyuan Zeng, Yang Zhao, Xinyi Xiang, Weifeng Su, Yaowen Xu, Sitaramanjaneya Mouli Thalluri, Fei Lin, Lifeng Liu; "Lead-doped ruthenium-iridium oxide catalysts for durable acidic oxygen evolution in proton exchange membrane electrolyzers at 3 A/cm2"; Materials Futures, 2025-11-3






























































