Resistance makes waves
There is a growing understanding of the conditions required for superconductivity and how it can be achieved at realistic temperatures
Advertisement
Even physics can give pointers for energy saving. An international team working together with the Centre for Quantum Materials, run by the Max Planck Society in conjunction with the University of British Columbia (Canada), is now in a position to provide materials scientists with tips for the development of high-temperature superconductors, in a bid to make them earn their name. The term is currently used to describe materials including ceramic cuprates, which lose their electrical resistance at significantly higher temperatures than conventional superconductors, but still well below the freezing point of water. In two complementary studies, the physicists have now established that superconductivity in cuprates collapses at a maximum of minus 135 degrees Celsius due to the formation of charge-density waves. These periodic fluctuations in the distribution of the electrical charges destroy superconductivity. Consequently, in order to find superconductors that drop to zero resistance at realistic temperatures, materials scientists must search for substances that are not subject to charge-density waves.
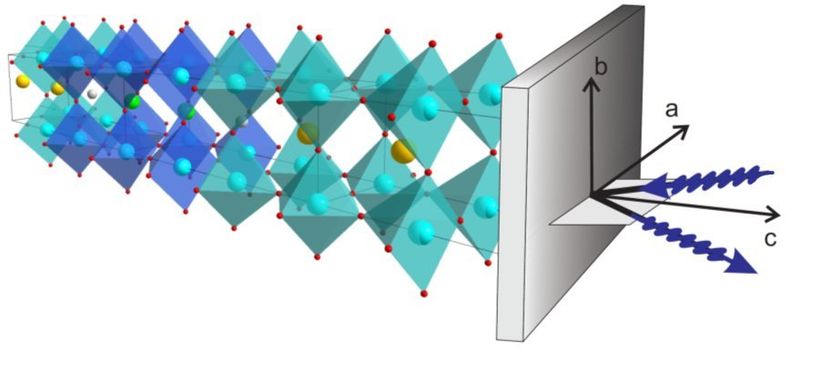
A look inside a high-temperature superconductor: The Max Planck scientists in Stuttgart used resonant X-ray scattering to show the existence of charge-density waves inside the cuprate superconductors. The blue wavy lines indicate the incident and emitted radiation. The system of coordinates illustrates the orientation of radiation relative to the crystal axes a, b and c.
© MPI for Solid State Research
Nearly two per cent of the electrical energy generated by power stations is lost in the grid. In Germany alone, this is equivalent to the power delivered by a medium-sized coal power plant. These losses may increase in the future, as power from large offshore wind farms is transported to the landlocked south. Superconductors could provide a remedy if they were able to deliver power to consumers without loss or leakage, even in summery temperatures. In order to systematically search for such materials, however, physicists must first obtain an accurate picture of why today's best superconductors lose resistance in the first place, and how the temperature at which this happens can be raised - a puzzle researchers have been working on for some 30 years. Little by little, a picture is starting to emerge. Two studies by an international team involving the Max Planck Institute for Solid State Research, as well as the universities of Princeton and British Columbia and the Helmholtz-Zentrum Berlin, have now contributed a few more pieces to the puzzle.
"We have found charge-density waves in cuprates above the temperatures at which they become superconductive", says Bernhard Keimer. "Like superconductivity, these are caused by strong interactions between the electrons." The Director at the Max Planck Institute for Solid State Research in Stuttgart was involved directly in one of the two studies, and in an advisory capacity in the other.
Contest for states decided by a hair’s breadth
Physicists have known for years that superconductivity can only arise in the first place if there is strong interaction between electrons. The fact is, forces – which current research assumes to be magnetic forces – bind electrons together to form Cooper pairs and these whizz through the crystal lattice unchecked. Researchers have also known for years that the strong interaction can induce other electronic phenomena such as magnetism or even charge-density waves, which are completely incompatible with superconductivity.
"These different states compete with each other in the materials", explains Keimer. "And which one wins is frequently decided by a mere hair’s breadth." This means that whether a material is superconductive or not depends to a very high degree on its elementary composition and its structure, while chance also gets in on the act. Still, the current studies are giving the scientists more of a feeling for when and in what circumstances superconductivity occurs. "We are getting closer to the goal of predicting this state and developing materials that will be superconductors even at high temperatures", says the physicist.
The international team is now contributing to a better understanding of superconductivity with experiments on two materials that contain the characteristic components of copper oxide and bismuth, and which are named Bi2201 and Bi2212 in accordance with the different proportions of elements they contain. The scientists studied a single sample of each material using different methods. In conjunction with a working group from Helmholtz-Zentrum Berlin, the Stuttgart-based Max Planck researchers screened both materials with resonant X-ray scattering using BESSY, the Helmholtz synchrotron. These experiments revealed details of the charge distribution on the inside of the materials. One of the participating scientists then travelled to Princeton University carrying the hermetically sealed material in a case. There, the project partners scanned the sample with a raster tunnel microscope that records the charge distribution at the surface. Physicists at the University of British Columbia also examined the Bi2201 sample using angle-resolved photoemission spectroscopy, which reveals further details of the electronic structure at the surface of the material.
Charge-density waves occur in all cuprate superconductors
Using the complementary examinations, the scientists demonstrated for both samples that the charge waves occur in different bismuth cuprates, and that they occur throughout the material and not just at the surface. "Since we have already detected the charge-density waves in another cuprate superconductor, we can assume that they occur in all cuprate superconductors and destroy superconductivity", affirms Bernhard Keimer.
One of the two studies has led scientists to complete a different part of the puzzle of high-temperature superconductivity, enabling them to explain anomalies in the band structure of these materials. The band structure is a kind of master plan of a material's electronic behaviour and can be read to determine whether the material is a metallic conductor, an insulator or a semiconductor. It reflects whether electrons are solidly bound, whether they can move freely through the material or whether they need an energy boost to get over a band gap in order to move freely.
The goal: precise control of the strong electronic forces
The band structure of superconductors contains pseudogaps, so called because, unlike the gaps in insulators, these gaps are incomplete and don't even exist for electrons at certain speeds. For many electrons, however, a pseudogap means that the charged particle can no longer move unhindered through the material. "We have now discovered that the cause of the pseudogap lies in the charge-density waves", explains Bernhard Keimer. This is easy to understand, because when electrons take on a fixed order, they lose their mobility. "So ultimately, pseudogaps can also be traced back to the strong interactions between electrons", adds Keimer.
In future, then, efforts will focus on precise control of the strong interactions between electrons. Only this will enable physicists and materials scientists to channel the forces in such a way that they cement Cooper pairs even at ambient temperatures and do not generate charge-density waves. "If we can manage that, we will have made an important contribution to the power supply of the future", says Bernhard Keimer.
Original publication
R. Comin, A. Frano, et al., Charge order driven by Fermi-arc instability in Bi2Sr2−xLaxCuO6+δ Science express, 19 December 2013
Eduardo H. da Silva Neto, Pegor Aynajian, Ubiquitous Interplay between Charge Ordering and High-Temperature Superconductivity in Cuprates Science express, 19 December 2013
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!