Smart Fluorescent Dyes
Modulating photo- and electroluminescence in a stimuli-responsive molecular dye
Advertisement
Controlling the excited electronic states in luminescent systems remains a challenge in the development of fluorescent and phosphorescent dyes. Now, scientists in Japan have developed a unique organic fluorophore that changes its emission color without loss of efficiency when externally stimulated. The study explains this behavior by a simple phase transformation of the solid substance, which could be relevant for optoelectronic applications such as in smart OLEDs.

© Wiley-VCH
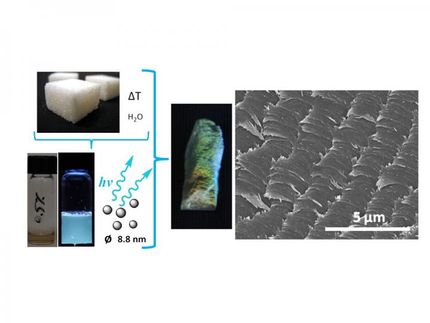
Although luminescence is an extensively studied phenomenon and its theoretical basis is well understood, the development of new pigments and dyes with outstanding functionality is not straightforward. Phase transitions of a solid material may quench the fluorescence, and pigments in OLED applications are prone to aging. Now, the research group of Takuma Yasuda at Kyushu University, Fukuoka, Japan, has synthesized a green emitting pigment that responds to external stimuli by a remarkable color change into orange emission, and that without no observed loss in luminescence efficiency. This two-color behavior of one pigment might by highly useful for the development of smart optoelectronic and sensor systems.
To obtain efficient luminescent systems, scientists are increasingly focusing on the excited states and the electronic transitions: The more distinct and defined the electronic transitions are, the more efficient is the light emission when the substance is excited by light of other wavelengths or electric energy. On the other hand, disturbances of the molecular structure can trigger nonradiative relaxation, and then, most fluorescence is lost. Here, Yasuda and his group found that their synthesized fluorophore, which has an elongated and relatively simple symmetric structure incorporating well-known chromophores, can switch its emission colors between orange and green when changing solid-state morphologies.
The authors substantiated their findings with X-ray crystallographic analyses and theoretical calculations. They found that the amorphous phase holds a slightly relaxed excited state compared to the crystalline one. This was explained by a twist in the molecule, which occurred at a different angle when the crystal structure was broken. Accordingly, the light emitted from that amorphous-phase excited state was at a longer wavelength than that emitted from the excited crystalline state.
Such a two-color emission from different solid phases could be useful for sophisticated optoelectronic and sensor applications. The Japanese authors found that the substance emitted orange fluorescence when deposited as a thin film, but this color turned to green when the film was annealed, that is, kept at high temperature and cooled down again. Then they scratched the annealed film and found orange fluorescence exactly at the places of scratching; even writing words in orange fluorescence was possible.
A more demanding application is that in organic light-emitting devices, the OLEDs. Sandwiched in an OLED setup, the compound exhibited bright electroluminescence, either in green when in the crystalline phase or in orange color when in the amorphous phase. This two-color electroluminescence from one pigment might be highly interesting for the ongoing research on stimuli-responsive smart materials.
Original publication
Kohei Isayama, Dr. Naoya Aizawa, Dr. Jun Yun Kim, Prof. Dr. Takuma Yasuda; "Modulating Photo‐ and Electroluminescence in a Stimuli‐Responsive π‐Conjugated Donor–Acceptor Molecular System"; Angew. Chem.; 2018
Kohei Isayama, Dr. Naoya Aizawa, Dr. Jun Yun Kim, Prof. Dr. Takuma Yasuda; "Modulating Photo‐ and Electroluminescence in a Stimuli‐Responsive π‐Conjugated Donor–Acceptor Molecular System"; Angew. Chem. Int. Ed.; 2018
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.