Stepwise self-organization of molecules into hexameric rosettes and then into nanotubes
05-Jan-2004
Tiny structures like nanoscopic tubes are highly desirable as potential
components for optoelectronics, "intelligent" materials, or the basis for new
systems for pharmaceutical transport in the body. Such mini tubes must have
precisely defined dimensions and specific chemical properties, which is not at
all easy to achieve. A Dutch-Belgian team led by Frans C. De Schryver and E. W.
Meijer has now synthesized a type of molecule that aggregates into long tubes
through stepwise self-organization.
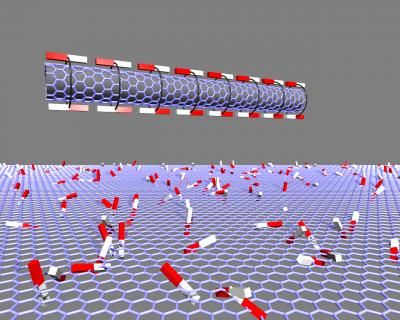
When the scientists created their molecules, two oligo para phenylvinylenes
(OPV3 and OPV4), they were actually not even doing research with nanotubes in
mind. Scanning tunneling microscopic images then revealed a surprise: having
been deposited on a Graphite support, the molecules arranged themselves into a
highly organized rosette-shaped structure. In solution, on the other hand, these
rosettes stack themselves into long tubes. The secret behind these unusual
aggregates lies in the special structure of the molecules, which consist of a
"head", a stretched "backbone", "arms" protruding from the sides, and three long
"tails". Six molecules at a time stick their heads together, and this social
circle is held together by two bridging hydrogen bonds between each pair of
neighboring heads. The arrangement of the bridges forces the heads into an
angled position, so that the backbones don't stick out like rays, but rather
form a rosette. The two-dimensional variation is fixed by the interlacing of the
tails of neighboring rosettes. Like a spiral, rosettes can be "twisted" either
clockwise or counterclockwise. Amazingly, OPV3 and OPV4 rosettes are twisted in
opposite directions, despite the fact that they only differ in the length of
their "backbone" and the number of arms (two or four). "The molecules are trying
to use the available space as intensively as possible -- without having bits of
molecule get in the way," explains Meijer. "Our OPVs are not completely
symmetrical and the two possible directions of rotation for the rosettes are
thus not equivalent. Which is preferred depends on the actual size and geometry
of the OPV in question. In addition, interactions with the graphite structure of
the support also play a role."
Stacking of the rosettes in solution, which are held together by attractive
forces between the flat aromatic rings of the OPV backbone, also involves
optimal use of space. This results in very dense tubes with an inner diameter of
about 1nm, which could be of interest as transport channels. Variation of the
OPVs should make it possible to obtain specific tubes with different dimensions
and properties.
Most read news
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.